Abstract
We designed and recombined the polypeptide based on the M protein of group A streptococci (GAS)—the causative pathogen of rheumatic fever and rheumatic heart disease, which would be a divalent vaccine to prevent and defend the diseases in relation to the different GAS strains. A divalent vaccine comprising three different peptide epitopes of the antiphagocytic M protein of GAS—an aminoterminal specific sequences, respectively, from the M1 and M12 proteins and J14 peptide (ASREAKKQVEKALE) within the highly conserved C-terminal repeat region of the M1 and M12 proteins—was subcutaneously delivered to mice with the adjuvant. Furthermore, the antisera titers of mice inoculated with the divalent vaccine were assayed by ELISA, and then opsonization and percentage killing against two different GAS serotypes were completed. Our data demonstrated that antisera raised against the divalent vaccine containing amino acids and M-protein-conserved C repeat region are able to kill several GAS strains isolated from the Guangzhou population. Therefore, the divalent vaccine can be used to prevent those diseases caused by GAS in an endemic area. We successfully construct the M-protein-based divalent vaccine that can bring out a high-level antisera titer of mice vaccinated with it. So, the vaccine has the potential to be used to prevent diseases caused by GAS in our country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group A streptococcus (GAS) is a human bacterial pathogen that colonizes throat or skin surfaces of the host. The infections of group A streptococci can elicit a spectrum of human diseases varying from the benign pharyngitis, tonsillitis, and pyoderma to the potentially life-threatening invasive diseases and serious nonsuppurative sequelae, including acute rheumatic (RF) and rheumatic heart disease (RHD) and acute glomerulonephritis rheumatic; while GAS is an important reason for morbidity and mortality in the world, the burden associated with GAS infectious diseases and serial complications is greater in developing countries and in indigenous populations of developed countries [1–3]. Thus, therapy for those illnesses needs new ways to develop the vaccine against GAS, except antibiotics. Most strategies of GAS vaccine have concentrated on the M protein, which is the antiphagocytic bacterial surface protein and has α helical coiled-coil construction with a high changeable amino terminal followed by three repeat regions called A, B and C repeats, a cell wall anchor motif and a stretch of hydrophobic amino acids, which are anchored in the cell membrane, and is an excellent candidate antigen to prevent GAS infections and RF/RHD. Previous studies have shown that M protein is the main virulence determinant and the important protective antigen of group A streptococci [5, 6]. The type specificity of M protein with more than 150 serotypes is mainly determined by the epitopes located in the amino domain containing 30–50 residues [4, 7–10]. These fragments of M proteins have been certified to evoke antibodies with the powerful bactericidal activity and have least possibility of interacting with human tissues [9, 11–15].
However, a main obstruction in the development of an M-protein-based vaccine for more than eight decades [16, 17] is the universal diversity of popular GAS strains and M protein types. Added to this is the possibility of evoking autoimmunity after inoculation as a result of molecular mimicry between the M protein and host tissue proteins. Addition, type-specific antibodies are not enough to provide more protection against multiple different GAS serotypes. Approaches employed to develop a broad strain coverage GAS vaccine have included the multivalent constructs containing type-specific M protein sequences [15, 18, 19] and the identification of vaccine candidates based on the conserved C region of the M protein [20–23]. Many important studies have been carried out for the potential of the M protein C-repeat region, which is conserved in different GAS strains as a vaccine candidate [20–23]. Using GenBank database search and in some investigations [24, 25], it has been found that about 60 % of GAS contain J14 sequences, while the remaining contain J14-like sequences. Now molecular techniques [4] and new biological studies of group A streptococci [5] have allowed the previous threshold associated with M protein vaccine development to be conquered.
In this study, we constructed a divalent vaccine based on M protein by recombinant technology. The vaccine comprised three different parts of M1 and M12 proteins including two episodes of 35 amino acids after signal peptide and J14 fragment linked in tandem. Our experiments showed the vaccine was highly immunogenic in mice delivered in complete Freund’s adjuvant and evoked widely protective antibody, as determined by opsonization and indirect bactericidal activity assays. Our results also demonstrated the feasibility of using component multivalent GAS M-protein-based vaccine to evoke benign antibody.
Materials and methods
Group A streptococcal strains
The parent type 1 and 12 streptococcal strains have been the subject of previous studies in our laboratory [26] and isolated from human tonsil or throat samples (The Third Affiliated Clinical Hospital of Sun Yat-sen University, Guangzhou, China) and identified by sequencing and alignment with streptococcal strains previously described in database (CDC and NCBI gene bank) [26].
Standards for selection of M peptides
The M1 and M12 serotypes were selected because each was represented by a substantial number of different subtypes. Its amino terminals (about 35 amino acids after signal peptide) and J14 peptide, which locates in the highly conserved C terminal of the selected M proteins, were searched by BlastP for homology against human proteins in the GenBank database. Amino-terminal regions having five or more contiguous amino acid matches with human proteins were excluded.
Construction and expression of the divalent fusion gene
Briefly, the designed hybrid DNA from emm1 and emm12 gene type of coding M1 and M12 protein of GAS was cloned and amplified by overlap PCR with 12 primers containing BamHI and XholI restriction sites at 5′ and 3′ terminals, respectively. The purified PCR product was digested with BamHI and XholI and inserted into T vector, which was sequenced. Later, both the T vector and the plasmid pGEX-4T-1 (Amersham Biosciences) were cut by BamHI and XholI, and the completed hybrid DNA molecules were ligated into pGEX-4T-1 to generate the pGEX-4T-1-emm1/12-J14(pE) expression vector used to transform Escherichia coli strain BL21. The integrity of the PCR-generated sequences was verified by sequencing both strands by the ABI dye termination method. Expression of GST fusion protein was detected by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) analysis using whole-cell lysates before and after isopropyl-β-d-thiogalactopyranoside (IPTG) induction.
Purification of the divalent vaccine
Harvested cells from E. coli BL21 liquid cultures expressed recombinant GST-tagged protein (GST/emm) by centrifugation at 10,000×g for 10 min. Decant supernatant, allow cell pellet to drain completely, and resuspend cell pellet on ice in BugBuster® Protein Extraction Reagent (BugBuster® GST·Bind Purification Kit, Novagen) for 1 h by pipetting. After centrifugation at 16,000×g for 20 min at 4 °C, remove insoluble cell debris, transfer supernatant to a fresh tube. Soluble extract (including GST fusion protein, GST/emm) was applied directly to GST·Bind™ Resin (Novagen). Fractions of eluted GST fusion protein (GST/emm) were monitored by SDS-PAGE and Western blot analysis with anti-GST monoclonal antibody(Cell Signaling Technology), and then the above fusion protein was cleaved by Thrombin (Thrombin Cleavage Capture Kit, Merk) and monitored by Coomassie Blue–stained 16 % Tricine-SDS-PAGE gels. Fractions containing both purified and cleaved protein were pooled and dialyzed against PBS before storage at −20 °C.
Immunization of mice
The female BALB/c mice (5–6 weeks old, Animal Resources Centre, SUN YAT-SEN UNIVERSITY) were randomly divided into two groups (n = 5, per group): emm group and normal group (control group). The divalent vaccine (emm) was administered subcutaneously in a volume of 50 μL to mice in the emm group. Each mouse received a total of 20 μg of immunogen emulsified 1:1 in complete Freund’s adjuvant (CFA) (Sigma). Mice were also given subsequent booster injections at days 21, 31, and 41 post-primary immunization with the recombinant peptide emulsified 1:1 in incomplete Freund’s adjuvant (IFA) (Sigma), while mice in control group also received injection with the mixture of adjuvant (CFA or IFA) and PBS.
Serum collection
Blood was collected from mice via the tail artery and allowed to clot at 37 °C for at least 30 min. Serum was collected after centrifugation at 1,000×g for 10 min, heat-inactivated at 56 °C for 10 min, and stored at −20 °C. Sera were collected from all of the mice 1 day prior to the first injection (as negative control serum, also belonged to normal group) and 14 days after the final boost.
Antibody assays
The purified recombinant divalent peptide (emm) was used as solid-phase antigens. Peptide was diluted to 10 μg/mL in carbonate coating buffer (pH 9.6) and coated onto polyvinyl plates (CORNING) in a volume of 0.1 mL/well overnight at 4 °C. The peptide was removed and the wells blocked with 0.1 mL of 5 % skim milk PBS–Tween 20 for 2 h at 37 °C. The plates were then washed three times with PBS–Tween 20 buffer. Mouse antisera were serially diluted in 0.5 % skim milk PBS–Tween 20 buffer, starting at an initial dilution of 1:1,000 to a final dilution of 1:7,000. Each sample was diluted to a final volume of 100 μL and incubated for 1.5 h at 37 °C. The plates were washed five times and peroxidase conjugated goat anti-mouse IgG (SATACRUZ) added at a dilution of 1:10,000 in 0.5 % skim milk PBS–Tween 20 for 1.5 h at 37 °C. After washing, 100 μL of ophenylenediamine (OPD) substrate (Sigma) was added according to the manufacturers’ instructions and incubated at room temperature for 30 min in the dark. The absorbance was measured at 450 nm in an ELISA plate reader. The highest dilution that gave an optical density (OD) 3 times higher than those of the average of control wells containing normal mouse serum at the same dilution was defined as the titer.
Opsonization assays
Opsonophagocytic antibodies for each of the M serotypes contained in the vaccine were measured prevaccination and 14 days following the last administration by using an assay previously published [12]. Briefly, The test mixture consisted of 0.05 mL of a diluted suspension (105) of streptococci grown to mid-log phase, 0.05 mL of test serum, and 0.2 mL of whole, heparinized (10 U/mL), nonimmune human blood, resulting in approximately 10 streptococcal colony-forming units (CFUs) per leukocyte in the mixture. After the entire mixture was rotated end-over-end at 37 °C for 45 min, smears were prepared on microscope slides that were air-dried and stained with Wright stain (Sigma Diagnostics). Opsonization was quantitated by counting 50 consecutive neutrophils and calculating the percentage with associated streptococci (percent opsonization).
Indirect bactericidal tests
Murine anti-emm serum samples were assayed for their ability to opsonize GAS in vitro, as described elsewhere [12, 16]. In brief, M1 GAS and M12 GAS were respectively grown overnight at 37 °C in 5 mL of Todd-Hewitt broth (THB). GAS was then serially diluted to 105 in PBS. For each individual mouse, 50 μL of fresh heat-inactivated antiserum was mixed with 50 μL of the bacterial dilution and incubated for 20 min at room temperature. After the incubation, 400 μL of nonopsonic heparinized human donor blood was added. All donor blood was prescreened before the assay was done, to ensure that it could support the growth of the GAS strain to at least 32 times the inoculum in a 3-h incubation at 37 °C [11]. The mixtures were incubated end-over-end at 37 °C for 3 h, and 50 μL from each tube was plated out in duplicate on 2 % blood THB agar pour plates. The plates were incubated overnight, and the number of colonies on each plate was determined. The results were expressed as percent killing, which was calculated by using the following formula: [1 − (mean CFU in the presence of antipeptide serum)/(mean CFU in the presence of normal mouse serum)]/100.
Statistical analysis
Preimmunization or control group versus post-immunization percentage opsonization or percent killing for the divalent vaccine was compared by using the Student’s t tests; mean and the standard error of the mean were calculated using standard formulae. The log-rank or Mantel–Haenszel test was used to compare geometric mean log-transformed titers. P < .05 was considered significant.
Results
Sequence and purification of the divalent vaccine
The protein sequence encoded by the hybrid DNA molecule was again analyzed by BlastP to ensure that there was no significant homology with human proteins (Fig. 1). Hybrid DNA molecule containing emm1 and emm12 gene fragments linked in tandem by unique restriction enzyme sites. Synthesis and purification of the hybrid DNA molecule and ligation with pGEX-4T-1(pG) (Amersham Biosciences) to form pGEX-4T-1-EMM1/12-J14 (pE) expression vector were performed in former experiment (data not shown). The expression vector was transformed to E. coli BL21, which was induced with IPTG, and GST-tagged fusion protein was purified with GST bind resin and cleaved with thrombin (Figs. 2, 3, 4).
SDS-PAGE analysis of purification of the recombinant GST/emm expressed in the E. coli BL21 strain. Lane M prestained protein marker, Lane 1 purified GST/emm fusion protein, Lane 2 intracellular soluble protein fractions. The above were loaded on SDS-PAGE gels and stained with Compasses Brilliant Blue R250
Thrombin cleavage of soluble GST/emm fusion protein. 16 % Tricine-SDS-PAGE analysis shows the thrombin digestion products of 100 μg of GST/emm fusion protein carried out in a thrombin cleavage capture kit using 10 U thrombin units. Lane M protein marker; Lane 1 shows 15 μL of the thrombin cleavage products of GST/emm fusion protein containing GST/emm with incomplete cleavage and removed GST and recombinant peptide (emm); Lane 2 purified GST/emm fusion protein (100 μg) before thrombin cleavage; Lane 3 recombinant peptide (emm) (upon completion of thrombin digestion, the glutathione and thrombin were removed by GST™ bind resin and streptavidin agarose)
Characteristics of the divalent vaccine (emm) against GAS
The divalent group A streptococcal vaccine contains amino-terminal regions and J14 peptide from the highly conserved region of two different M types. The selection of M types for inclusion in this vaccine was based on epidemiologic data from our former research [26]. The overall goal was to include the majority of serotypes that are common causes of streptococcal pharyngitis [26] and the serotypes that are either currently associated or have historically been associated with ARF. In addition, very little sequence variation was observed within the type-specific regions of these types [15, 25].
Immunogenicity of the divalent vaccine (emm) in mice
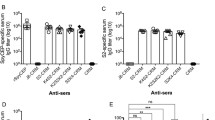
Vaccination elicited tenfold or more increases in ELISA assays. The geometric mean antibody titer after vaccination was significantly higher than the preimmune or control sera for the antigen measured in emm group. The experiments were performed to determine the immunogenicity of the vaccine in group of five mice that each received four subcutaneous injections of emm. ELISAs were performed with preimmune sera (obtained 1 day prior to the first injection) or normal sera and immune sera (obtained 2 weeks after the final injection) against emm. The immune sera from mice contained high titers of antibody against the divalent vaccine (Fig. 5). The preimmune or control titer was less than 100.
Type-specific M protein antibody evoked by the divalent vaccine (emm) in mice determined by ELISA. The average emm-specific serum IgG antibody titers of BALB/c mice, which the emm group was immunized with the mixture of divalent vaccine and adjuvant and the control group was injected with the mixture of adjuvant and PBS (seen “Materials and methods”). Booster immunizations (20 μg) were administered on days 21, 31 and 41 in two groups. The purified recombinant peptide evoked high serum antibody titers as the figure showed, but antibody titers of <100 were observed for all preimmune sera or the control sera when tested against the antigen. In other words, the immune sera contained significant antibody levels (serum antibody titers that increased by tenfold or greater over preimmune or control levels) against the divalent vaccine antigen
Opsonization of different strains of group A streptococci
To determine whether the divalent vaccine (the recombinant peptide, emm) evoked opsonic antibody against two serotypes of group A streptococci (obtained our previous surveillance, as above), opsonization assays were performed with antisera against the purified vaccine and designed to determine the percentage of neutrophils that engulfed or were associated with streptococci after rotation in nonimmune human blood that contained either preimmune (normal else) or immune mouse serum. Percentage opsonization of M1 and M12 serotypes was high in the immune serum (98.3 ± 0.51 and 95.3 ± 0.35, respectively), whereas very low level of opsonization was observed by preimmune or normal sera (<10 % opsonization of each of the two serotypes tested), indicating that the donor blood used for these assays did not contain antibodies against the test organism and that each organism was fully resistant to opsonization in nonimmune blood (Table 1; Fig. 6).
Opsonic activity of serum antibody evoked in mice by the divalent vaccine (emm). Immune sera from five mice were tested in in vitro opsonization assays using M1 and M12 serotypes of group A streptococci. Percent opsonization was the mean percentage of neutrophils engulfed or associated with streptococci (±SEM) following incubation in immune serum and was compared with that of either preimmune or control serum. Percent opsonization for each individual group was shown with the SE represented as an error bar. *Opsonization was statistically significantly higher (P < .05) compared with the preimmune serum (resulted in <10 % opsonization with each organism as well as normal serum; data not shown)
Bactericidal activity of the divalent antisera against type 2 streptococci
Antibody from mice immunized with the vaccine (emm) was also tested for its ability to kill M1 and M12 GAS in a bactericidal assay. As shown in Table 2 and Fig. 7, the level of opsonic activity displayed by emm antisera was over 90 % both in M1 and in M12 serotypes. Significant opsonization of the strains of M1 and M12 GAS was high in the presence of emm antiserum, which made CFU decrease 98.1 and 97.3 %, respectively (P < .05, emm group versus control group or preimmune sera). In bactericidal assays, each of the two serotypes of group A streptococci was rotated in nonimmune human blood for 3 h in the presence of either preimmune or immune mice serum (as seen “Materials and methods”). In all experiments, the test mixture containing preimmune serum resulted in growth of the organisms for eight or more generations (Table 2), again indicating that the human blood did not contain opsonic antibodies against the test strains and that each organism was fully resistant to bactericidal killing in nonimmune blood.
Opsonic activity of serum antibodies stimulated after subcutaneous delivery with the immunogen (emm). Opsonization of M1 and M12 group A streptococcus (GAS) serotypes by mice antisera was determined by a bactericidal assay. The growth of bacteria in colony-forming units (CFU) following incubation in the presence of immune serum was compared with that of control serum. The mean percentage of opsonization (measured as the percentage reduction in colony-forming units of bacteria) for each individual group was shown with the SE represented as an error bar. *Opsonization was statistically significantly higher (P < .001) compared with the control group
Discussion
As we know, due to GAS infections prevailing in some countries, the huge burden of economy and society is increasing day and day, in which those nonsuppurative sequela of group A streptococcal infections represent a severe health problem and the therapies are also poor or insufficient. Current available prevention methods largely focus on the specific vaccine against different GAS strains based on the M proteins. The basic evidence supporting the grounds for M-protein-based group A streptococcal vaccines was largely established by the prospective work of many scholars such as Rebecca Lancefield [6, 16, 27, 28] who first explained the type specificity of protective antibodies stimulated GAS in initial stages. Following studies in many other laboratories [16, 29–33] identified the amino acid sequences of several M proteins, which discovered the considerably varied structures in amino-terminal part of the M proteins that involved the type-specific, protective epitopes [9–11, 34, 35]. By only riveting on these determined regions of M proteins, the epitopes without tissue cross-reaction [8, 36, 37] could be excluded from multivalent vaccines. Molecular clone technology has made us successfully synthesis multivalent vaccines [13, 14]. In this study, we utilized the same techniques to produce a divalent vaccine.
M protein serotypes included in the divalent vaccine was selected on the basis of our present prospective surveillance of streptococcal sore throat in Guangzhou [26], in which bacteria isolated from GAS culture-positive sore throat children, were cultured and identified with NCBI database and defined in relationship with GAS-serotypes-caused ARF or AHD. In view of the abovementioned results, our current aim is to construct and develop a vaccine to fit our country that will decrease the total disease burden raised by group A streptococci in China. We also know that the huge overlap exists between different GAS serotypes that cause invasive disease or common infections, but only a few types of GAS cultures were discovered in our former experiments [26]. Thus, we used M1 and M12 proteins of GAS serotypes isolated and identified in current epidemiologic studies to build the divalent vaccine. In addition, we gave up some GAS stains preserved in our clinical laboratory because the cases of these GAS strains were insufficient for the vaccine construction. So, the M protein serotypes of GAS selected in the divalent vaccine were mainly based on present data that considered the future popular trends.
In the present study, mice were inoculated with 20 μg antigen (divalent vaccine). The vaccine contained 84 amino acids including amino parts of the M1 and M12 proteins and J14 peptide lying in the C-terminal region. J14 peptide included in the divalent vaccine represents the same sequences in the two types of M proteins, which also elicit opsonic antibodies [22–24]. In other words, this fusion protein held its own carrier, which makes it greatly possible that the antibody elicited will be functionally active. The immunogenicity of the divalent vaccine was analyzed by the enzyme-linked immunologic assay, opsonization assays, and bactericidal activity tests (seen the “Materials and methods”). The ELISA was performed with the divalent vaccine, and all five mice immunized with this vaccine evoked high levels of antibodies (more than fourfold titer increase) against the divalent antigens. Opsonization and indirect bactericidal activity assays were performed to define the function of the antisera by using the two different serotypes of group A streptococci. Mostly, there was a very good correlation between the results of all three assays. There was a direct correlation between the results of opsonization and bactericidal activity assays with the two serotypes tested (seen the “Results”). We concluded that a comprehensive analysis of the potential efficacy of M-protein-based vaccines should be based on the results of both assays performed with the same immune sera. Study showed a 20-amino-acid peptide from the amino terminal of one serotype of M protein can stimulate opsonized antibodies against a heterologous serotype [8, 10, 36]. The cross-opsonic antibodies did not cross-react with the amino-terminal peptide of the heterologous M protein, indicating that the cross-opsonic antibodies may recognize epitopes in other regions of M or M-like proteins [10]. In this research, it is also possible that the antibody evoked by amino-terminal peptides of M proteins cross-reacted with opsonic epitopes on those serotypes not represented in the vaccine. Studies are presently in progress to determine the nonvaccine types that may be cross-opsonized and the vaccine peptides that may evoke cross-reactive antibodies. As a result, antisera obtained from the mice injected with the antigen (emm) should provide a better protection against the two serotypes of group A streptococci. To some extent, the deficiency in our present experiments was that the coverage of GAS serotypes is lower than in other researches owing to fewer GAS strains isolated from local children affected by upper respiratory tract infection [26].
To conclude, our study demonstrated that antisera against the divalent vaccine containing specific amino acid sequences and partly conserved C-repeat region from M1 and M12 proteins are able to kill two different GAS serotypes isolated from Guangzhou children. We have also shown that a component divalent M-protein-based vaccine is immunogenic in mice and stimulates greatly opsonic antibody against the two vaccine types. We believe that this vaccine has the ability to induce broadly protective antibody responses in humans, which may decrease disease burden caused by group A streptococci.
References
Bisno AL, Rubin FA, Cleary PP et al (2005) Prospects for a group A streptococcal vaccine: rationale, feasibility, and obstacles–report of a National Institute of allergy and infectious diseases workshop. Clin Infect Dis 41:1150–1156
McMillan DJ, Chhatwal GS (2005) Prospects for a group A streptococcal vaccine. Curr Opin Mol Ther 7:11–16
Brandt ER, Good MF (1999) Vaccine strategies to prevent rheumatic fever. Immunol Res 19:89–103
Beall B, Facklam R, Thompson T (1995) Sequencing emm-specific polymerase chain reaction products for routine and accurate typing of group A streptococci. J Clin Microbiol 34:953–958
Cunningham MW (2000) Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511
Lancefield RC (1962) Current knowledge of the type specific M antigens of group A streptococci. J Immunol 89:307–313
Beachey EH, Seyer JM, Dale JB, Simpson WA, Kang AH (1981) Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature (Lond) 292:457–459
Dale JB, Beachey EH (1985) Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med 162:583–591
Dale JB, Beachey EH (1986) Localization of protective epitopes of the amino terminus of type 5 streptococcal M protein. J Exp Med 163:1191–1202
Jones KF, Manjula BN, Johnston KH, Hollingshead SK, Scott JR, Fischetti VA (1985) Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J Exp Med 161:623–628
Beachey EH, Seyer JM (1986) Protective and nonprotective epitopes of chemically synthesized peptides of the NH2-terminal region of type 6 streptococcal M protein. J Immunol 136:2287–2292
Dale JB, Seyer JM, Beachey EH (1983) Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J Exp Med 158:1727–1732
Dale JB (1999) Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 17:193–200
Dale JB, Simmons M, Chiang EC, Chiang EY (1996) Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 14:944–948
Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB (2002) Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun 70:2171–2177
Dochez AR, Avery OT, Lancefield RC (1919) Studies on the biology of Streptococcus. J Exp Med 30:179–213
Lancefield RC (1928) The antigenic complex of Streptococcus haemolyticus. 1. Demonstration of type-specific substance in extracts of Streptococcus haemolyticus. J Exp Med 47:91–103
Kotloff KL, Corretti M, Palmer K, Campbell JD, Reddish MA, Hu MC, Wasserman SS, Dale JB (2004) Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA 292:709–715
Hall MA, Stroop SD, Hu MC, Walls MA, Reddish MA, Burt DS, Lowell GH, Dale JB (2004) Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections. Infect Immun 72:2507–2512
Mannam P, Jones KF, Geller BL (2004) Mucosal vaccine made from live, recombinant Lactococcus lactis protects mice against pharyngeal infection with Streptococcus pyogenes. Infect Immun 72:3444–3450
Batzloff MR, Yan H, Davies MR, Hartas J, Lowell GH, White G, Burt DS, Leanderson T, Good MF (2005) Toward the development of an antidisease, transmission-blocking intranasal vaccine for group a streptococcus. J Infect Dis 192:1450–1455
Pruksakorn S, Galbraith A, Houghten RA, Good MF (1992) Conserved T and B cell epitopes on the M protein of group A streptococci. Induction of bactericidal antibodies. J Immunol 149:2729–2735
Bessen D, Fischetti VA (1990) Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J Immunol 145:1251–1256
Brandt ER, Hayman WA, Currie B, Pruksakorn S, Good MF (1997) Human antibodies to the conserved region of the M protein: opsonisation of heterologous strains of group A streptococci. Vaccine 15:1805–1812
Vohra H, Dey N, Gupta S, Sharma AK, Kumar R, McMillan D, Good MF (2005) M protein conserved region antibodies opsonise multiple strains of Streptococcus pyogenes with sequence variations in C-repeats. Res Microbiol 156:575–582
Ding Yue-xia, NI Q-q, Liu J-l (2011) Prospective surveillance of streptococcal sore throat in Guangzhou presently. J Sun Yat-Sen Univ 32:411–415
Lancefield RC (1957) Differentiation of group A streptococci with a common R antigen into three serologic types with special reference to the bactericidal test. J Exp Med 106:525–544
Lancefield RC (1959) Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med 110:271–292
Beachey EH, Seyer JM, Dale JB, Hasty DL (1983) Repeating covalent structure and protective immunogenicity of native and synthetic polypeptide fragments of type 24 streptococcal M protein. J Biol Chem 258:13250–13257
Beachey EH, Seyer JM, Kang AH (1980) Primary structure of protective antigens of type 24 streptococcal M protein. J Biol Chem 255:6284–6289
Miller L, Gray L, Beachey EH, Kehoe M (1988) Antigenic variation among group A streptococcal M proteins: nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding type 1, 6 and 24 M proteins. J Biol Chem 263:5668–5673
Fischetti VA, Jones KF, Manjula BN, Scott JR (1984) Streptococcal M6 protein expressed in Escherichia coli. Localization, purification and comparison with streptococcal-derived M protein. J Exp Med 159:1083–1095
Manjula BN, Seetharma-Acharya A, Mische SM, Fairwell T, Fischetti VA (1984) The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J Biol Chem 259:3686–3693
Dale JB, Chiang EC (1995) Intranasal immunization with recombinant group A streptococcal M protein fragment fused to the B subunit of Escherichia coli labile toxin protects mice against systemic challenge infections. J Infect Dis 171:1038–1041
Jones KF, Fischetti VA (1988) The importance of the location of antibody binding on the M6 protein for opsonization and phagocytosis ofgroup A M6 streptococci. J Exp Med 167:1114–1123
Baird RW, Bronze MS, Kraus W, Hill HR, Veasey LG, Dale JB (1991) Epitopes of group A streptococcal M protein shared with antigens of articular cartilage and synovium. J Immunol 146:3132–3137
Dale JB, Beachey EH (1985) Multiple heart-cross-reactive epitopes of streptococcal M proteins. J Exp Med 161:113–122
Acknowledgments
This work was supported by Foundation of Scientific and Technologic Committee of Guangdong Province, China (Grant no. 2008B080701023). We appreciate the expert technical assistance provided by Liu Wei. The Scientific and Technologic Committee of Guangdong Province, China.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, Y., Ni, Q., Liu, J. et al. Immunogenicity of a divalent group A streptococcal vaccine. Rheumatol Int 33, 1013–1020 (2013). https://doi.org/10.1007/s00296-012-2455-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2455-8