Abstract
The aim of the study was to test the frequency of CD4+ CD25highFoxP3 regulatory T cells in JIA patients and to assess their activation status and functional activity. The study involved 12 children with JIA and 35 healthy control subjects. PBMC were stained with monoclonal antibodies (anti-CD25, anti-CD4, anti-CD127, anti-CD69, anti-CD71, and anti-FoxP3). The samples were evaluated using flow cytometer. CD4+ CD25− and CD4+ CD25+ cells were isolated by negative and positive selection with magnetic microbeads. CD4+ CD25+ and CD4+ CD25− cells were cultured separately and co-cultured (1:1) with or without PHA. The percentage of Tregs in JIA patients was significantly decreased in comparison with controls (median, 3.2 vs. 4.6; P = 0.042). Relative fluorescence intensities of FoxP3 were higher in JIA patients than in controls (median, 9.1 vs. 6.8). The percentage of activated Tregs (CD71+) was significantly higher in JIA patients in comparison with controls (median, 6.5 vs. 2.8; P = 0.00043). CD4+ CD25+ cells derived from JIA patients and controls were anergic upon PHA stimulation, while CD4+ CD25− cells showed intensive proliferative response. The proliferation rate of CD4+ CD25− cells stimulated by PHA was decreased in co-cultures. In JIA patients, the inhibition of proliferation of CD4+ CD25− cells by CD4+ CD25+ cells was 37.9%, whereas in controls it was significantly lower (55.7%, P = 0.046). JIA patients had statistically lower percentage of Tregs in peripheral blood compared to controls. CD4+ CD25+ cells sorted from peripheral blood of JIA patients had statistically lower ability to suppress CD4+ CD25− cell proliferation in comparison with cells obtained from controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) belongs to the group of connective tissue diseases. This is the most common chronic autoimmune disease in children [1–4]. The etiology of this type of chronic arthritis in children is unknown. The incidence of JIA is assessed for approximately 2.6–13.9/100,000 children/year among Caucasian children below 15 years of age, with a prevalence of approximately 6.5–113/100,000 children [4].
In the Classification Criteria of the International League of Associations for Rheumatology (ILAR), JIA is regarded not as a single disease but as a category of diseases with three principal types of onset: (1) oligoarthritis or pauciarticular disease (OA), (2) polyarthritis (PA), and (3) systemic-onset disease (SO) [4]. Six distinct subtypes are identified. ILAR propose an additional definition and classification schema (age at onset <16 years; arthritis in ≥1 joints; duration of disease is ≥6 weeks; onset type defined by type of articular involvement in the first 6 months after onset: polyarthritis, oligoarthritis, systemic disease, psoriatic arthritis, enthesitis-related arthritis and undifferentiated arthritis, and exclusion of other forms of arthritis) [3, 5, 6]. Full pathogenesis of JIA is still a matter of investigation. Some already known reasons are genetic and environmental factors, infections, and also deregulation of immune system, which results in autoreactivity [3, 5].
To maintain peripheral tolerance, the immune system has evolved a number of regulatory mechanisms. Failure of this regulatory network can result in autoimmune disease development. The most important cells in the immune system regulation are CD4+ CD25high regulatory T (Tregs) cells. They can control autoagressive T cells and B cells, which somehow escape from negative selection in thymus. They can block activation and effector functions of autoreactive T cells [7–12].
In human, Tregs were identified in both peripheral blood and thymus. Only these CD4+ CD25+ T cells that express high levels of CD25 display regulatory functions [13, 14]. Tregs are divided into several populations. Naturally occurring Tregs, which express the forkhead transcription factor (FoxP3), are found in thymus and in peripheral blood, where they account for 5–10% of CD4+ T cells. FoxP3 is a crucial factor responsible for the development of Tregs in the thymus. It is also needed to maintain the suppressive activity of mature peripheral Tregs and is a specific molecular marker for Tregs in human peripheral blood. Decreased FoxP3 expression causes conversion of Tregs into effector cells. The last phenomenon can be responsible for autoimmune diseases development [15]. FoxP3 expression in Tregs plays a crucial role in maintaining immunological tolerance [16, 17].
There are suggestions that in rheumatoid arthritis (RA) and JIA, Tregs function is impaired. However, the studies concerning regulatory T cells in RA or JIA are rather ambiguous. In some papers the authors report decreased number of regulatory T cells in peripheral blood in patients with RA or JIA [12, 18, 19], whereas others show just the opposite—increased number of these cells [20] or no changes in Tregs number [21, 22]. Moreover, there are so many variances in data concerning Tregs in JIA and RA patients because of no specific criteria to identify these cells. Some authors analyzed whole population of CD4CD25 cells, but not exactly CD4CD25high. Some did not use FoxP3 expression as a specific marker for regulatory T cells [21–24].
As it is suggested by many authors, FoxP3 expression is crucial for the identification of cells bearing suppressive activity [25–27]. However, FoxP3 alone is not sufficient to mark off this specific subpopulation. The remaining Tregs’ antigens (for example, CD152 and GITR) are not expressed in the whole population. Solely, CD4 and high expression of CD25 are characteristic of all Tregs. However, the assessment and exact gating of cells with high expression of CD25 is very subjective and depend on the person who performs flow cytometry analysis or who analyze the results. In many situations, there is no distinct borderline between CD25low and CD25high populations. Some authors use additional factors such as CD3 and CD4 expression, which is a little lower on CD25high T cells [18]. Importantly, the population of CD4CD25high cells includes FoxP3 negative cells that are recognized as activated T cells. In this situation, the assessment of CD127 expression can be very useful [28–30]. According to Seddiki et al. [30] and Liu et al. [29], CD127 is downregulated in Tregs. A strong, statistically significant correlation between FoxP3 and CD127low was found in CD4CD25 T cells in humans. It provides a possibility to separate activated T cells, which are CD127 positive, from Tregs, which are CD127low or negative. Hence, CD127 can be useful marker used in the purification of CD4+ CD25highFoxP3 + T cells population.
According to Ruprecht et al. [31], CD27 can also be a good marker of Treg cells useful to distinguish them from conventional CD4+ cells. CD27 antigen is lost by these cells upon terminal differentiation [32] and as was proven by Ruprecht et al. [31], the combination of CD25 and CD27 antigens allows identifying most of Tregs (CD4+ CD25highCD27+).
Important activity of Tregs is their ability to suppress effector T cells and to limit their inflammatory function. It was documented that Tregs obtained from patients with RA are defective in their ability to suppress cytokine production (TNF-α, INF-γ) [21]; however, the results concerning this issue are controversial. Nevertheless, there is general agreement concerning one question: synovial fluid (SF) of JIA patients contains high number of Tregs [12, 23, 31, 33].
Activation status of Tregs is unclear. Some authors report lack of activation markers expression (e.g., CD69 or CD71) on Tregs’ surface [34], but the others documented their expression on Tregs [13] or even increased CD69 expression after specific therapy [35]. Cao et al. [33] described the presence of CD71 antigen on PMBC and SF Tregs in patients with RA.
Taking all these facts into consideration, the aim of this study was not only to test the frequency but also to assess the activation status and functional activity of CD4+ CD25highFoxP3 regulatory T cells in JIA patients.
The study was approved by Independent Ethics Committee of the Medical University of Warsaw.
To perform proliferation assays, we needed to collect at least 8 ml of peripheral blood to assure the separation of sufficient number of cells used for co-cultures. Due to ethical reasons, we included only 14-year-old or older children (n = 7) in the study. Blood collected from healthy adults served as a control, because the immunological systems of such children and adults are very similar.
Material
Patients
The study involved 12 children with JIA (treated in the Department of Pediatrics, Medical Centre of Postgraduate Education, Warsaw, and Clinic and Polyclinic of Rheumatology of Developmental Age, Institute of Rheumatology, Warsaw), 29 healthy children, and 6 healthy adult volunteers. Table 1 shows the clinical characteristics of children with JIA and healthy controls.
The patients with JIA were in active phase of disease and fulfilled the classification criteria of the ILAR from Durban 1997 published by Petty et al. [3]. None of the patients was treated with glicocorticosteroids, because prednisolone or other drugs from this group may affect lymphocyte functions and CD25 expression [36].
Methods
From young children with JIA and healthy control children, 0.5–2.0 ml of heparinized blood was collected from antecubital vein. From children over 14 years old and from adult volunteers, 8 ml of heparinized blood was collected.
Cells
Peripheral blood mononuclear cells (PBMC) were isolated using a standard Ficoll-Histopaque®-1077 (Sigma–Aldrich Co., St. Louis, USA) gradient centrifugation (Sigma Diagnostic Instruction Manual). Cells’ concentration was adjusted to 1 × 106/ml in control culture medium (RPMI1640 supplemented with 10% FCS, 200 mM l-glutamine, and 1 ml of 1 M HEPES). The procedures were performed in sterile conditions. Viability of blood lymphocytes was determined by trypan blue staining and achieved approximately 95–98%.
Proceedings of FoxP3 expression in CD4+ CD25highCD127− T cells examination, and the assessment of CD4+ CD25high T cells activation
Freshly isolated 100 μl of 1 × 106/ml PBMC was stained with 5 or 10 μl of monoclonal antibodies (according to manufacturer’s instructions, BD-Pharmingen) in 2 tubes:
-
tube no. 1—anti-CD25 PE-Cy7, clone M-A251; anti-CD4 PE-Cy-5; and anti-CD127 PE
-
tube no.2—anti-CD69 PE; anti-CD71 FITC; anti-CD25 PE-Cy7, clone M-A251; and anti-CD4 PE-Cy-5.
Both tubes were incubated for 20 min in the dark at room temperature. Cells were washed twice in washing buffer (PBS supplemented with 0.5% inactivated FBS) for 5 min, 250 g.
FoxP3 intracellular staining was performed in tube 1 according to manufacturer’s instruction (BD-Pharmingen). Briefly, cells were incubated for 10 min in the dark at room temperature with 2 ml of buffer A, then washed in washing buffer for 5 min, 500 g, and incubated for 30 min in 500 μl of buffer C in the dark at room temperature. Subsequently, the cells were stained with 20 μl anti-FoxP3 monoclonal antibody (Alexa Flour 488, BD-Pharmingen) for 30 min in the dark at room temperature and washed twice.
Flow cytometric analysis
The appropriate isotype controls were included. The samples were evaluated within 24 h on Cytomics FC500 flow cytometer (Beckmann Coulter).
Cell isolation and selection
CD4+ cells were isolated from freshly isolated PBMC by negative selection according to manufacturer’s protocol (MACS, MiltenyiBiotech). Briefly, PBMC were incubated for 20 min in 4°C with biotin monoclonal antibodies cocktail (10 μl/107 cells) against: CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ, and CD235a (MiltenyiBiotech). Then, the cells were incubated for 15 min in 4°C with antibiotin-labeled microbeads (20 μl/107 cells) and washed twice in washing buffer (10 min, 300 g) and depleted using MACS LD column.
The obtained CD4+ T cells were washed twice in washing buffer (10 min, 300 g) resuspended and labeled with 10 μl of anti-CD25 microbeads (10 μl/107 cells). Subsequently, the cells were incubated for 15 min in 4°C. After the incubation, 10 μl of anti-CD25 PE monoclonal antibody and 5 μl of anti-CD4FITC (BD-Pharmingen) monoclonal antibodies were added. The cells were then incubated for the next 15 min at 4°C and washed twice in washing buffer. Then CD4+ T cells were positively selected using MACS MS column according to manufacturer’s protocol (MACS, MiltenyiBiotech).
After the last centrifugation, the selected subpopulations of CD4+ CD25− and CD4+ CD25+ T cells were resuspended in culture medium (RPMI 1640 supplemented with 10% FCS, l-Glutamine 200 mM, HEPES 1 M, antibiotic–antimycotic) and were immediately used in proliferation assay.
Proliferation assay
We cultured 1 × 105 of the selected CD4+ CD25− or CD4+ CD25+ T cells, resuspended in 100 μl of culture medium, in sterile plastic 96-well, flat-bottom microwell culture plates–Nunclon (Nunc A/S, Denmark) at 37°C at 95% humidity and in an atmosphere containing 5% CO2.
For proliferation analysis, CD4+ CD25+ and CD4+ CD25− T cells were cultured separately, 100 μl of control medium with or without phytohaemagglutinin (PHA) (Sigma–Aldrich) was added to the appropriate wells. The final concentration of PHA was 20 μg/ml.
For suppression analysis CD4+ CD25− and CD4+ CD25+ T cells were co-cultured at 1:1 ratio (0.5 × 105 + 0.5 × 105) in the same conditions.
After 54 h of culture, 1 μCi/well 3H-thymidine was added for the remaining 18 h of culture. Cells were harvested using cell harvester (Skatron, Norway). Proliferation was measured using a liquid scintillation counter LKB Wallac 1409 (RackBeta LKB, Sweden).
For proliferation inhibition analysis, the CD4+ T cells purified from peripheral blood were separated into CD25− and CD25+ populations and subsequently cultured with or without PHA.
Proliferation index was counted as follows:(cmp of 3[H] Thymidine incorporation of cells cultured with PHA–cmp of 3[H] Thymidine incorporation of cells cultured without PHA)/cmp of 3[H] Thymidine incorporation of cells cultured without PHA
Proliferation index of 1 × 105 cells was divided into 2 to get a proliferation index of 0.5x105.
Percentage of inhibition of proliferative response was counted as follows:
Statistical analysis
All of the measured parameters had nonparametric distribution (according to Shapiro–Wilk’s criteria), so statistical analysis was performed using nonparametric Wilcoxon test for dependent samples and nonparametric Mann–Whitney U test for independent samples. To assess the correlations between results, Spearman’s test was used.
Results
Percentages of CD4+ CD25highFoxP3 + regulatory T cells in peripheral blood of JIA patients and healthy controls
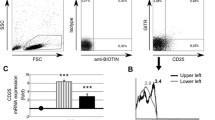
Tregs in peripheral blood were identified as CD4+ CD25+ highFoxP3 + CD127− T cells. The number of regulatory T cells is expressed as a percentage of all CD4+ T cells.
As shown in Fig. 1, the percentage of Tregs in JIA patients was significantly decreased in comparison with healthy controls (median (25 percentile; 75 percentile): 3.2 (2.09; 4.78) vs. 4.6 (3.61; 5.81), respectively, P = 0.042).
All JIA patients suffered from one of two subtypes of JIA: OA (n = 5) and PA (n = 7). No differences between the percentages of Tregs in peripheral blood of the above-mentioned groups of patients were found (median (25 percentile; 75 percentile): OA 4.4 (1.89; 5.55), PA 2.7 (2.29; 3.58)).
Relative fluorescence intensities (RFI) of FoxP3 expression were also compared. RFI was calculated using the following formula: experimental mean fluorescence intensity (MFI)/MFI with isotype control antibody according to Dechant et al. [37]. We saw higher RFI of FoxP3 expression in JIA patients than in healthy controls (median (25 percentile; 75 percentile): 9.1 (7.24; 11.22), 6.8 (5.59; 9.51), respectively). The results are shown in Fig. 2.
The comparison of total population of CD4+ CD25+ T cells in JIA patients and healthy controls did not reveal any differences (about 14% of CD4 T cells were CD25 positive). Additionally, the percentage of lymphocytes (median (25 percentile; 75 percentile): 36.0 (28.50; 46.00), 42.7 (31.40; 52.50) and the percentage of CD4FoxP3 cells (median (25 percentile; 75 percentile): 6.4 (2.87; 8.48), 6.8 (5.70; 9.19)) were not different.
Activation status of CD4+ CD25high T cells–expression of CD69 and CD71 on CD4+ CD25high T cells
In the performed experiments, we assessed CD71 and CD69 expression on CD4+ CD25high T cells without analyzing FoxP3 or CD127 expression. Fig. 3 shows the percentages of activated Tregs, which express CD71 antigen on the surfaces. We found significantly higher expression of this antigen on Tregs from JIA patients than in healthy controls (median (25 percentile; 75 percentile): 6.5 (3.83; 13.13) vs. 2.8(1.47; 4.31), respectively, P = 0.00043).
When we compare CD69 expression on Tregs, the percentages of activated Tregs were similar in JIA patients and in healthy controls (median (25 percentile; 75 percentile): (3.5 (1.30; 6.04) vs. 3.1(1.90; 4.34), respectively).
Inhibition of proliferation of CD4+ CD25− T cells by CD4+ CD25+ T cells
CD4+ CD25+ T cells derived from peripheral blood of JIA patients and healthy controls were anergic upon PHA stimulation (proliferation index: −0.36 (−0.39; 0.05), 0.04 (−0.41; 0.35), respectively), CD4+ CD25− T cells showed very intensive proliferative response (proliferation index: 78.7 (47.68; 382.79), 87.0 (62.68; 168.23), respectively).
Proliferation of CD4+ CD25− T cells stimulated by PHA was decreased in cultures when CD4+ CD25+ T cells were added in 1:1 ratio (proliferation index: 55.2 (40.61; 244.07) for JIA and 35.7 (28.94; 53.24) for controls).
In peripheral blood of JIA patients, the inhibition of proliferation of CD4+ CD25− cells by CD4+ CD25+ T cells was 37.9%, and it was significantly lower in comparison with healthy controls (55.7%, P = 0.046). The results of this experiment are shown in Fig. 4.
Individual inhibition of proliferation of CD4+ CD25− T cells by CD4+ CD25+ T cells collected from peripheral blood of JIA patients (n = 7) and healthy controls (n = 6). Cultures were stimulated with PHA for 72 h, proliferation was measured for last 18 h using [3H] incorporation. Counting formula is shown in Methods section
Discussion
In our study, we identified Tregs as CD4+ CD25highFoxP3 + CD127− T cells. It means that we classified CD4CD25high T cells as Tregs only when they showed FoxP3 expression and were negative for CD127 expression. In our JIA group, we saw significantly lower percentage of Tregs in peripheral blood than in control group. We are aware that our JIA patient group was small but SD was quite small, which let us to obtain significant results. Our findings are similar to that published by Kleer et al. [12] that concerned bigger group of JIA patients (n = 60 and n = 34) as well as the results of studies performed by Cao et al. [24] (n = 165, but in this paper Foxp3 was not assessed) or recently by Olivito et al. [38] (n = 65).
There are several explanations for the decreased percentage of Tregs in JIA patients. One of them is that during inflammatory process, which occurs in JIA, Tregs are attracted to the sites of inflammation, especially to the affected joints. That is the reason for the decreased number of Tregs in peripheral blood. Many authors observed increased infiltration of functional Tregs in synovial fluid aspirated from affected joints [12, 18, 22–24, 33, 39]. Of course, Tregs can proliferate in joints. Unfortunately, we did not have the opportunity to examine synovial fluid from affected joints.
Our results obtained from co-culture experiments showed that not only was the number of Tregs in JIA limited but also their suppressive activity was reduced. Median inhibition of proliferation of CD4+ CD25− T cells in JIA patients was 37.9%. Moreover in some patients, low suppressive activity (to 2.9 and 6.6%) was observed, while in healthy controls, the mean value was 55.67% with the lowest suppressive activity achieved, i.e., 43.3%. We did not observe any correlation between clinical features and the level of proliferation inhibition in JIA patients.
Because both groups in the performed study were relatively small and the dispersion level was quite big, it was really hard to use any statistic method.
This finding is in agreement with some others results, however, showing significant decrease in suppressive capacity of Tregs or just the tendency [20, 22]. The remaining authors did not find any decrease in suppressive activity of Tregs from RA or JIA patients [12, 18, 21]. Low suppressive capacity may be caused by the interaction between antigen-presenting cells (APCs) and Tregs, which is altered by overexpression of co-stimulatory molecules (CD80, CD86, CD40) on APCs. Such mechanism was described by van Amelsdorf et al. [22] for synovial fluid monocytes. In rheumatoid diseases the interaction between Tregs and effector T cells was also affected. CD4+ CD25− T cells were less sensitive to suppression by Tregs [12, 22].
Cao et al. [24] showed that CD25brightCD4+ T cells, known as activated memory T cells, express CD71. RA patients, in comparison with healthy controls, showed higher intensity of the CD71 staining in CD25brightCD4+ population. In a paper published by Havryluk et al. [40], the authors presented significantly increased expression of CD71 on peripheral blood lymphocytes than in healthy control group; however, the study did not focus on Tregs. In our results, Tregs obtained from JIA patients showed higher expression of this activation marker than that from healthy control’s Tregs. Our patients were in active phase of the disease—4 of them (25%) were at the onset of the disease, most of them had high ESR level (9/12, 75%) and CRP concentration in serum (5/12, 42%). We consider these results cautiously, because we did not check FoxP3 expression in the same tube as the expression of CD71, CD127, CD4, and CD25 antigens.
Not only the criteria of identification, but also the degree, duration, and subtype of JIA can influence Tregs status. In our study, we showed the data obtained from patients with OA and PA. The comparison of result obtained for these two groups did not reveal any differences; however, as we mention above, our groups were relatively small. De Kleer et al. [12] showed that persistent OA JIA patients had significantly higher number of CD4+ CD25bright Treg in peripheral blood than extended OA JIA patients. Lawson et al. [18] observed that in the peripheral blood of early active RA patients comparably smaller proportion of CD4+ CD25high T cells was found than in patients with reactive arthritis.
In our study, we focus on cells that apart from CD4 and CD25high were positive for FoxP3 and negative for CD127. According to these criteria, the patients with JIA had statistically lower percentage of Tregs in peripheral blood comparing to healthy controls. We also showed that CD4CD25high cell sorted from peripheral blood of JIA patients have statistically lower ability to suppress CD4CD25− T cells proliferation in comparison with cells obtained from healthy controls. Better identification and characterization of Tregs in JIA is needed, because the decrease in the number of these cells may lead to downregulation of T cells tolerance and exacerbate the inflammatory processes. Some authors postulate that abnormal T-cell regulation may underline the persistent inflammation characteristic of rheumatoid diseases [18].
References
Woo O, Wedderburn LR (1998) Juvenile rheumatoid arthritis. Lancet 351:969–973
Wallace CA, Levinson JE (1991) Juvenile rheumatoid arthritis: outcome and treatment for the 1990 s. Rheum Dis Clin North Am 17:891–905
Petty RE, Sounthwood TR, Manners P (2004) International Leauge of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J Rheumatol 31:390–392
Hayward K, Wallace CA (2009) Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Research & Therapy 11:216–227
Ravelli A, Martini A (2007) Juvenile idiopathic arthritis. Lancet 369:767–778
Cassidy JT, Levison JE, Bass JC et al (1986) A study of classification criteria for a diagnosis of juvenile rheumatoid arthritis. Arthritis Rheum 29:174–181
Dieckmann D, Plottner H, Berchtold S et al (2001) Ex Vivo Isolation and Characterization of CD4+ CD25+ T Cells with Regulatory Properties from Human Blood. J Exp Med 193:1303–1310
Jonuleit H, Schmitt E, Stassen M et al (2001) Identification and Functional Characterization of Human CD4+ CD25+ T Cells with Regulatory Properties Isolated from Peripheral Blood. J Exp Med 193:1285–1294
Levings MK, Sangregorio R, Sartirana C (2002) Human CD25+ CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 196:1335–1346
Baecher-Allan C, Viglietta V, Hafler DA (2004) Human CD4+ CD25+ regulatory T cells. Semin Immunol 16:89–97
Taams LS, Vukmanovic-Stejic M, Smith J (2002) Antigen-specific T cell suppression by human CD4+ CD25+ regulatory T cells. Eur J Immunol 32:1621–1630
Kleer IM, Wedderburn LR, Taams LS et al (2004) CD4+ CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol 172:6435–6443
Baecher-Allan C, Wolf E, Hafler DA (2005) Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+ CD25+ T cells. Clin Immunol 115:10–18
Beacher-Allan C, Brown JA, Freemann GJ et al (2001) CD4+ CD25high regulatory cells in human peripheral blood. J Immunol 167:1245–1253
Williams LM, Rudensky AY (2007) Maintenance of the FoxP3-dependent developmental program in mature regulatory T cells requires continued expression of FoxP3. Nat Immunol 8:277–284
Vignali DA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8:523–532
Wan YY, Flavell RA (2007) Regulatory T-cell functions are subverted and converted owing to attenuated FoxP3 expression. Nature 45:766–770
Lawson CA, Brown AK, Bejarano V et al (2006) Early rheumatoid arthritis is associated with a deficit in the CD4+ CD25high regulatory T cell population in peripheral blood. Rheumatology 45:1210–1217
Wei C-M, Lee J-H, Wang L-C (2008) Frequency and phenotypic analysis of CD4+ CD25+ regulatory T cells in children with juvenile idiopathic arthritis. J Microbiol Immunol Infect 41:78–87
Han GM, O’Neil-Andersen NJ, Zurier RB, Lawrence DA (2008) CD4+ CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cel Immunol 253:92–101
Ehrenstein MR, Evans JG, Singh A et al (2004) Compromised Function of Regulatory T Cells in Rheumatoid Arthritis and Reversal by Anti-TNF Therapy. J Exp Med 200:277–285
Amelsfort JMR, Jacobs KMG, Bijlsma JWJ et al (2004) CD4+ CD25+ regulatory T cells in Rheumatoid Arthritis. Arthritis&Rheumatism 50:2775–2785
Mottonen M, Heikkinen J, Mustonen L et al (2005) CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol 140:360–367
Cao D, Malmstrom V, Beacher-Allan C et al (2003) Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 33:215–223
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol 4:330–336
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Khattri R, Cox T, Yasayko S-A et al (2003) An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol 4:337–342
Banham AH (2006) Cell-surface IL-7 receptor expression facilitates the purification of FOXP3 + regulatory T cells. Trends in Immunol 27:541–544
Liu W, Putnam AL, Xu-yu Z (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203:1701–1711
Seddiki N, Santner-Nanan B, Martinson J (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203:1693–1700
Ruprecht CR, Gattorno M, Ferlito F et al (2005) Coexpression of CD25 and CD27 identifies FoxP3 + regulatory T cells in inflamed synovia. J Exp Med 201:1793–1803
de Jong R, Brouwer M, Hooibrink B (1992) The CD27- subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol 22:993–999
Cao D, van Vollenhoven R, Klareskog L et al (2004) CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther 6:R335–R346
Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E (2002) Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology 106:190–199
Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL (2006) Characterization of CD4+ CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 24:1169–1177
Lamas M, Sanz E, Martin-Parras L et al (1993) Glucocorticoid hormones upregulate interleukin 2 receptor alpha gene expression. Cell Immunol 151:437–450
Dechant M, Weisner W, Berger S et al (2008) Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res 68:4998–5003
Olivito B, Simonini G, Ciullini S et al (2009) Th17 transcription factor RORC2 is inversely correlated with FoxP3 expression in the joints of children with juvenile idiopathic arthritis. J Rheumatol 36:2017–2024
Nistala K, Moncrieffe H, Newton KR et al (2008) Interleukin-17–Producing T Cells Are Enriched in the Joints of Children With Arthritis, but Have a Reciprocal Relationship to Regulatory T Cell Numbers. Arthritis&Rheumatism 58:875–887
Havryluk A, Bilyy R, Tolstiak J (2009) Evaluation of immunological criteria for rheumatoid arthritis. Centr Eur J Immunol 34:176–181
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Stelmaszczyk-Emmel, A., Jackowska, T., Rutkowska-Sak, L. et al. Identification, frequency, activation and function of CD4+ CD25highFoxP3+ regulatory T cells in children with juvenile idiopathic arthritis. Rheumatol Int 32, 1147–1154 (2012). https://doi.org/10.1007/s00296-010-1728-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-010-1728-3