Abstract
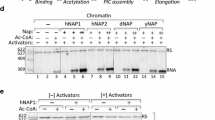
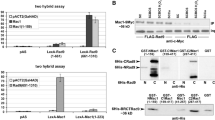
To gain insights on the transcriptional switches that modulate proper copper homeostasis in yeast, we have examined in detail functional interactions of the relevant transcriptional activator Mac1. We identified Hir1 transcriptional repressor and histone chaperone as a Mac1-interacting protein. This association directly recruits Hir1 on a Mac1 target, CTR1 promoter, quantitatively under induction conditions. We also found Hir1 interacting directly with a previously unknown partner, the Ssn6 (Cyc8) co-regulator. On the non-induced CTR1 promoter, a Hir1 transcriptional activation function was revealed, in the absence of Ssn6, which was dependent on the presence of Snf2 (Swi2) nucleosome remodeler. Moreover, Ssn6 was identified as a Mac1-dependent prominent repressor of CTR1 transcription, antagonizing Snf2 occupancy. Transcriptional induction by copper depletion was effected by the quantitative recruitment of Snf2 directed mainly by Mac1 and redundantly by the quantitatively accumulated Hir1 and Ssn6 pair. Our analysis showed that the activation-effecting chromatin remodeling of CTR1 was due to Snf2 and not to the Hir1 histone chaperone activity or ability to regulate histone levels and stoichiometry. Following initiation, Hir1 and Snf2, but not Ssn6, were found to associate also with the actively transcribing CTR1 coding region, where Hir1 followed the pattern of the elongating RNA polymerase II. Therefore, we have shown that, at the CTR1 gene, in association with Mac1 DNA-binding transcriptional activator, the distinct and alternate genetic and physical collaboration of three global regulators modulates the transcriptional state of a switch involved in copper homeostasis.









Similar content being viewed by others
References
Ahmad A, Kikuchi H, Takami Y, Nakayama T (2005) Different roles of N-terminal and C-terminal halves of HIRA in transcription regulation of cell cycle-related genes that contribute to control of vertebrate cell growth. J Biol Chem 280(37):32090–32100. https://doi.org/10.1074/jbc.M501426200
Amin AD, Vishnoi N, Prochasson P (2013) A global requirement for the HIR complex in the assembly of chromatin. Biochim Biophys Acta 1819(3–4):264–276. https://doi.org/10.1016/j.bbagrm.2011.07.008
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1987–2003) Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York
Becker DM, Fikes JD, Guarente L (1991) A cDNA encoding a human CCAAT-binding protein cloned by functional complementation in yeast. Proc Natl Acad Sci USA 88(5):1968–1972
Belotserkovskaya R, Saunders A, Lis JT, Reinberg D (2004) Transcription through chromatin: understanding a complex FACT. Biochim Biophys Acta 1677(1–3):87–99. https://doi.org/10.1016/j.bbaexp.2003.09.017
Bereketoglu C, Arga KY, Eraslan S, Mertoglu B (2017) Analysis of transcriptional profiles of Saccharomyces cerevisiae exposed to bisphenol A. Curr Genet 63(2):253–274. https://doi.org/10.1007/s00294-016-0633-z
Bilsland E, Molin C, Swaminathan S, Ramne A, Sunnerhagen P (2004) Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol Microbiol 53(6):1743–1756
Chujo M, Tarumoto Y, Miyatake K, Nishida E, Ishikawa F (2012) HIRA, a conserved histone chaperone, plays an essential role in low-dose stress response via transcriptional stimulation in fission yeast. J Biol Chem 287(28):23440–23450. https://doi.org/10.1074/jbc.M112.349944
Ciftci-Yilmaz S, Au WC, Mishra PK, Eisenstatt JR, Chang J, Dawson AR, Basrai MA (2018) A genome-wide screen reveals a role for the HIR histone chaperone complex in preventing mislocalization of budding yeast CENP-A. Genetics. https://doi.org/10.1534/genetics.118.301305
Collins SR, Kemmeren P, Zhao XC, Greenblatt JF, Spencer F, Holstege FC, Krogan NJ (2007) Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol Cell Proteom 6(3):439–450. https://doi.org/10.1074/mcp.M600381-MCP200
Conlan RS, Gounalaki N, Hatzis P, Tzamarias D (1999) The Tup1–Cyc8 protein complex can shift from a transcriptional co-repressor to a transcriptional co-activator. J Biol Chem 274(1):205–210
Corey LL, Weirich CS, Benjamin IJ, Kingston RE (2003) Localized recruitment of a chromatin-remodeling activity by an activator in vivo drives transcriptional elongation. Genes Dev 17(11):1392–1401. https://doi.org/10.1101/gad.1071803
De Freitas J, Wintz H, Kim JH, Poynton H, Fox T, Vulpe C (2003) Yeast, a model organism for iron and copper metabolism studies. Biometals 16(1):185–197
DeRisi JL, Iyer VR, Brown PO (1997) Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278(5338):680–686
DeSilva H, Lee K, Osley MA (1998) Functional dissection of yeast Hir1p, a WD repeat-containing transcriptional corepressor. Genetics 148(2):657–667
Dimova D, Nackerdien Z, Furgeson S, Eguchi S, Osley MA (1999) A role for transcriptional repressors in targeting the yeast Swi/Snf complex. Mol Cell 4(1):75–83
Eriksson PR, Ganguli D, Nagarajavel V, Clark DJ (2012) Regulation of histone gene expression in budding yeast. Genetics 191(1):7–20. https://doi.org/10.1534/genetics.112.140145
Field LS, Luk E, Culotta VC (2002) Copper chaperones: personal escorts for metal ions. J Bioenerg Biomembr 34(5):373–379
Fillingham J, Kainth P, Lambert JP, van Bakel H, Tsui K, Pena-Castillo L, Andrews BJ (2009) Two-color cell array screen reveals interdependent roles for histone chaperones and a chromatin boundary regulator in histone gene repression. Mol Cell 35(3):340–351. https://doi.org/10.1016/j.molcel.2009.06.023
Formosa T, Ruone S, Adams MD, Olsen AE, Eriksson P, Yu Y, Stillman DJ (2002) Defects in SPT16 or POB3 (yFACT) in Saccharomyces cerevisiae cause dependence on the Hir/Hpc pathway: polymerase passage may degrade chromatin structure. Genetics 162(4):1557–1571
Fragiadakis GS, Tzamarias D, Alexandraki D (2004) Nhp6 facilitates Aft1 binding and Ssn6 recruitment, both essential for FRE2 transcriptional activation. Embo J 23(2):333–342
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev 106(6):1995–2044. https://doi.org/10.1021/cr040410w
Georgakopoulos T, Koutroubas G, Vakonakis I, Tzermia M, Prokova V, Voutsina A, Alexandraki D (2001) Functional analysis of the Saccharomyces cerevisiae YFR021w/YGR223c/YPL100w ORF family suggests relations to mitochondrial/peroxisomal functions and amino acid signalling pathways. Yeast 18(12):1155–1171
Georgatsou E, Alexandraki D (1999) Regulated expression of the Saccharomyces cerevisiae Fre1p/Fre2p Fe/Cu reductase related genes. Yeast 15(7):573–584
Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D (1997) The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J Biol Chem 272(21):13786–13792
Graden JA, Winge DR (1997) Copper-mediated repression of the activation domain in the yeast Mac1p transcription factor. Proc Natl Acad Sci USA 94(11):5550–5555
Green EM, Antczak AJ, Bailey AO, Franco AA, Wu KJ, Yates JR III, Kaufman PD (2005) Replication-independent histone deposition by the HIR complex and Asf1. Curr Biol 15(22):2044–2049. https://doi.org/10.1016/j.cub.2005.10.053
Grove A (2018) Control of RNA polymerase II-transcribed genes by direct binding of TOR kinase. Curr Genet 64(1):131–135. https://doi.org/10.1007/s00294-017-0738-z
Gutierrez JL, Chandy M, Carrozza MJ, Workman JL (2007) Activation domains drive nucleosome eviction by SWI/SNF. EMBO J 26(3):730–740. https://doi.org/10.1038/sj.emboj.7601524
Jensen LT, Winge DR (1998) Identification of a copper-induced intramolecular interaction in the transcription factor Mac1 from Saccharomyces cerevisiae. Embo J 17(18):5400–5408
Jenull S, Tscherner M, Gulati M, Nobile CJ, Chauhan N, Kuchler K (2017) The Candida albicans HIR histone chaperone regulates the yeast-to-hyphae transition by controlling the sensitivity to morphogenesis signals. Sci Rep. https://doi.org/10.1038/s41598-017-08239-9
Kaufman PD, Cohen JL, Osley MA (1998) Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol Cell Biol 18(8):4793–4806
Keller G, Bird A, Winge DR (2005) Independent metalloregulation of Ace1 and Mac1 in Saccharomyces cerevisiae. Eukaryot Cell 4(11):1863–1871
Kim HJ, Seol JH, Han JW, Youn HD, Cho EJ (2007) Histone chaperones regulate histone exchange during transcription. EMBO J 26(21):4467–4474. https://doi.org/10.1038/sj.emboj.7601870
Kliewe F, Engelhardt M, Aref R, Schuller HJ (2017) Promoter recruitment of corepressors Sin3 and Cyc8 by activator proteins of the yeast Saccharomyces cerevisiae. Curr Genet 63(4):739–750. https://doi.org/10.1007/s00294-017-0677-8
Knop M, Siegers K, Pereira G, Zachariae W, Winsor B, Nasmyth K, Schiebel E (1999) Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15(10B):963–972
Kuo MH, Allis CD (1999) In vivo cross-linking and immunoprecipitation for studying dynamic protein:DNA associations in a chromatin environment. Methods 19(3):425–433
Kusuya Y, Hagiwara D, Sakai K, Yaguchi T, Gonoi T, Takahashi H (2017) Transcription factor Afmac1 controls copper import machinery in Aspergillus fumigatus. Curr Genet 63(4):777–789. https://doi.org/10.1007/s00294-017-0681-z
Labbe S, Zhu Z, Thiele DJ (1997) Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem 272(25):15951–15958
Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, Young RA (2002) Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298(5594):799–804
Loyola A, Almouzni G (2004) Histone chaperones, a supporting role in the limelight. Biochim Biophys Acta 1677(1–3):3–11
Magnaghi P, Roberts C, Lorain S, Lipinski M, Scambler PJ (1998) HIRA, a mammalian homologue of Saccharomyces cerevisiae transcriptional co-repressors, interacts with Pax3. Nat Genet 20(1):74–77
Malave TM, Dent SY (2006) Transcriptional repression by Tup1-Ssn6. Biochem Cell Biol 84(4):437–443
Martins LJ, Jensen LT, Simon JR, Keller GL, Winge DR (1998) Metalloregulation of FRE1 and FRE2 homologs in Saccharomyces cerevisiae. J Biol Chem 273(37):23716–23721
Mason PB, Struhl K (2003) The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol 23(22):8323–8333
Mei Q, Huang J, Chen W, Tang J, Xu C, Yu Q, Li S (2017) Regulation of DNA replication-coupled histone gene expression. Oncotarget 8(55):95005–95022. https://doi.org/10.18632/oncotarget.21887
Neely KE, Hassan AH, Brown CE, Howe L, Workman JL (2002) Transcription activator interactions with multiple SWI/SNF subunits. Mol Cell Biol 22(6):1615–1625
Nourani A, Robert F, Winston F (2006) Evidence that Spt2/Sin1, an HMG-like factor, plays roles in transcription elongation, chromatin structure, and genome stability in Saccharomyces cerevisiae. Mol Cell Biol 26(4):1496–1509. https://doi.org/10.1128/MCB.26.4.1496-1509.2006
Osley MA, Gould J, Kim S, Kane MY, Hereford L (1986) Identification of sequences in a yeast histone promoter involved in periodic transcription. Cell 45(4):537–544
Papamichos-Chronakis M, Petrakis T, Ktistaki E, Topalidou I, Tzamarias D (2002) Cti6, a PHD domain protein, bridges the Cyc8-Tup1 corepressor and the SAGA coactivator to overcome repression at GAL1. Mol Cell 9(6):1297–1305
Peterson CL, Dingwall A, Scott MP (1994) Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc Natl Acad Sci USA 91(8):2905–2908
Prochasson P, Florens L, Swanson SK, Washburn MP, Workman JL (2005) The HIR corepressor complex binds to nucleosomes generating a distinct protein/DNA complex resistant to remodeling by SWI/SNF. Genes Dev 19(21):2534–2539. https://doi.org/10.1101/gad.1341105
Proft M, Struhl K (2002) Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol Cell 9(6):1307–1317
Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6(2):171–180
Ray-Gallet D, Quivy JP, Scamps C, Martini EM, Lipinski M, Almouzni G (2002) HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol Cell 9(5):1091–1100
Rees EM, Thiele DJ (2004) From aging to virulence: forging connections through the study of copper homeostasis in eukaryotic microorganisms. Curr Opin Microbiol 7(2):175–184
Ricketts MD, Marmorstein R (2017) A molecular prospective for HIRA complex assembly and H3.3-specific histone chaperone function. J Mol Biol 429(13):1924–1933. https://doi.org/10.1016/j.jmb.2016.11.010
Roberts SM, Winston F (1997) Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics 147(2):451–465
Rustici G, van Bakel H, Lackner DH, Holstege FC, Wijmenga C, Bahler J, Brazma A (2007) Global transcriptional responses of fission and budding yeast to changes in copper and iron levels: a comparative study. Genome Biol 8(5):R73
Rutherford JC, Bird AJ (2004) Metal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cells. Eukaryot Cell 3(1):1–13
Saunders A, Core LJ, Lis JT (2006) Breaking barriers to transcription elongation. Nat Rev Mol Cell Biol 7(8):557–567. https://doi.org/10.1038/nrm1981
Schermer UJ, Korber P, Horz W (2005) Histones are incorporated in trans during reassembly of the yeast PHO5 promoter. Mol Cell 19(2):279–285
Schwabish MA, Struhl K (2007) The Swi/Snf complex is important for histone eviction during transcriptional activation and RNA polymerase II elongation in vivo. Mol Cell Biol 27(20):6987–6995. https://doi.org/10.1128/MCB.00717-07
Serpe M, Joshi A, Kosman DJ (1999) Structure-function analysis of the protein-binding domains of Mac1p, a copper-dependent transcriptional activator of copper uptake in Saccharomyces cerevisiae. J Biol Chem 274(41):29211–29219
Sharp JA, Fouts ET, Krawitz DC, Kaufman PD (2001) Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr Biol 11(7):463–473
Sharp JA, Franco AA, Osley MA, Kaufman PD (2002) Chromatin assembly factor I and Hir proteins contribute to building functional kinetochores in S. cerevisiae. Genes Dev 16(1):85–100
Sherwood PW, Tsang SV, Osley MA (1993) Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol Cell Biol 13(1):28–38
Singer RA, Johnston GC (2004) The FACT chromatin modulator: genetic and structure/function relationships. Biochem Cell Biol 82(4):419–427
Smith RL, Johnson AD (2000) Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem Sci 25(7):325–330
Soontorngun N (2017) Reprogramming of nonfermentative metabolism by stress-responsive transcription factors in the yeast Saccharomyces cerevisiae. Curr Genet 63(1):1–7. https://doi.org/10.1007/s00294-016-0609-z
Spector MS, Osley MA (1993) The HIR4-1 mutation defines a new class of histone regulatory genes in Saccharomyces cerevisiae. Genetics 135(1):25–34
Spector MS, Raff A, DeSilva H, Lee K, Osley MA (1997) Hir1p and Hir2p function as transcriptional corepressors to regulate histone gene transcription in the Saccharomyces cerevisiae cell cycle. Mol Cell Biol 17(2):545–552
Svejstrup JQ (2003) Transcription. Histones face the FACT. Science 301(5636):1053–1055
Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y (2004) Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116(1):51–61
Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Boone C (2004) Global mapping of the yeast genetic interaction network. Science 303(5659):808–813
Tzamarias D, Struhl K (1995) Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8–Tup1 corepressor complex to differentially regulated promoters. Genes Dev 9(7):821–831
Van Ho A, Ward DM, Kaplan J (2002) Transition metal transport in yeast. Annu Rev Microbiol 56:237–261
van Bakel H, Strengman E, Wijmenga C, Holstege FC (2005) Gene expression profiling and phenotype analyses of S. cerevisiae in response to changing copper reveals six genes with new roles in copper and iron metabolism. Physiol Genom 22(3):356–367
Voutsina A, Fragiadakis GS, Boutla A, Alexandraki D (2001) The second cysteine-rich domain of Mac1p is a potent transactivator that modulates DNA binding efficiency and functionality of the protein. FEBS Lett 494(1–2):38–43
Wong KH, Struhl K (2011) The Cyc8–Tup1 complex inhibits transcription primarily by masking the activation domain of the recruiting protein. Genes Dev 25(23):2525–2539. https://doi.org/10.1101/gad.179275.111
Yamaguchi-Iwai Y, Serpe M, Haile D, Yang W, Kosman DJ, Klausner RD, Dancis A (1997) Homeostatic regulation of copper uptake in yeast via direct binding of MAC1 protein to upstream regulatory sequences of FRE1 and CTR1. J Biol Chem 272(28):17711–17718
Yoon S, Qiu H, Swanson MJ, Hinnebusch AG (2003) Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA. Mol Cell Biol 23(23):8829–8845
Zhang H, Gan H, Wang Z, Lee JH, Zhou H, Ordog T, Zhang Z (2017) RPA interacts with HIRA and regulates H3.3 deposition at gene regulatory elements in mammalian cells. Mol Cell 65(2):272–284. https://doi.org/10.1016/j.molcel.2016.11.030
Acknowledgements
We thank Mary Ann Osley for LexA-N-Hir1 and LexA-C-Hir1 derivatives, Tim Formosa for spt16-11, pob3-7 strains and Fred Winston for snf2 strain. We thank Ioannis Kagiambakis for help with a control Mac1-9Myc experiment. We thank the late George Thireos, the late Yannis Papanikolau, Dimitris Tzamarias, Iannis Talianidis, Ioannis Kagiampakis, George Voloudakis and Metaxia Vlassi for helpful discussions on the subject. We thank Marianthi Kiparaki, Panagiota Spiliotopoulou and Katerina Bakela for help with the experiments.
Funding
This work was supported by the Greek Ministry of Development—GSRT (IMBB funding and PENED Grants 99EΔ 417 and 01ED119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The authors declare that (a) the research presented in this manuscript does not contain any fabricated or manipulated data and has not been submitted to another journal and (b) they have no conflict of interest.
Additional information
Communicated by M. Kupiec.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Voutsina, A., Fragiadakis, G.S., Gkouskou, K. et al. Synergy of Hir1, Ssn6, and Snf2 global regulators is the functional determinant of a Mac1 transcriptional switch in S. cerevisiae copper homeostasis. Curr Genet 65, 799–816 (2019). https://doi.org/10.1007/s00294-019-00935-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00935-5