Abstract
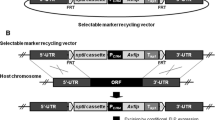
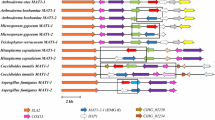
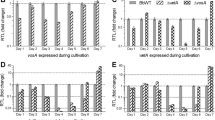
Most superficial fungal infections are caused by dermatophytes, a specialized group of filamentous fungi which exclusively infect keratinized host structures such as hair, skin and nails. Since little is known about the molecular basis of pathogenicity and sexual reproduction in dermatophytes, here we functionally addressed two central transcriptional regulators, SteA and StuA. In the zoophilic species Arthroderma benhamiae a strategy for targeted genetic manipulation was recently established, and moreover, the species is teleomorphic and thus allows performing assays based on mating. By comparative genome analysis homologs of the developmental regulators SteA and StuA were identified in A. benhamiae. Knock-out mutants of the corresponding genes as well as complemented strains were generated and phenotypically characterized. In contrast to A. benhamiae wild type and complemented strains, both mutants failed to produce sexual reproductive structures in mating experiments. Analysis of growth on keratin substrates indicated that loss of steA resulted in the inability of ΔsteA mutants to produce hair perforation organs, but did not affect mycelia formation during growth on hair and nails. By contrast, ΔstuA mutants displayed a severe growth defect on these substrates, but were still able to produce hair perforations. Hence, formation of hair perforation organs and fungal growth on hair per se are differentially regulated processes. Our findings on the major role of SteA and StuA during sexual development and keratin degradation in A. benhamiae provide insights into their role in dermatophytes and further enhance our knowledge of basic biology and pathogenicity of these fungi.





Similar content being viewed by others
References
Ajello L, Cheng SL (1967) The perfect state of Trichophyton mentagrophytes. Sabouraudia 5:230–234
Aramayo R, Peleg Y, Addison R, Metzenberg R (1996) Asm-1 +, a Neurospora crassa gene related to transcriptional regulators of fungal development. Genetics 144:991–1003
Asunción Garcia-Sánchez M, Martín-Rodrigues N, Ramos B, de Vega-Bartol JJ, Perlin MH, Díaz-Mínguez JM (2010) Fost12, the Fusarium oxysporum homolog of the transcription factor Ste12, is upregulated during plant infection and required for virulence. Fungal Genet Biol 47:216–225. doi:10.1016/j.fgb.2009.11.006
Borneman AR, Hynes MJ, Andrianopoulos A (2001) An STE12 homolog from the asexual, dimorphic fungus Penicillium marneffei complements the defect in sexual development of an Aspergillus nidulans steA mutant. Genetics 157:1003–1014
Borneman AR, Hynes MJ, Andrianopoulos A (2002) A basic helix-loop-helix protein with similarity to the fungal morphological regulators, Phd1p, Efg1p and StuA, controls conidiation but not dimorphic growth in Penicillium marneffei. Mol Microbiol 44:621–631
Burmester A, Shelest E, Glöckner G, Heddergott C, Schindler S, Staib P, Heidel A, Felder M, Petzold A, Szafranski K, Feuermann M, Pedruzzi I, Priebe S, Groth M, Winkler R, Li W, Kniemeyer O, Schroeckh V, Hertweck C, Hube B, White TC, Platzer M, Guthke R, Heitman J, Wöstemeyer J, Zipfel PF, Monod M, Brakhage AA (2011) Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genome Biol 12:R7. doi:10.1186/gb-2011-12-1-r7
Chou S, Lane S, Liu H (2006) Regulation of mating and filamentation genes by two distinct Ste12 complexes in Saccharomyces cerevisiae. Mol Cell Biol 26:4794–4805. doi:10.1128/mcb.02053-05
Clutterbuck AJ (1969) A mutational analysis of conidial development in Aspergillus nidulans. Genetics 63:317–327
Deng F, Allen TD, Nuss DL (2007) Ste12 transcription factor homologue CpST12 is down-regulated by hypovirus infection and required for virulence and female fertility of the chestnut blight fungus Cryphonectria parasitica. Eukaryot Cell 6:235–244. doi:10.1128/ec.00302-06
Dinamarco TM, Almeida RS, de Castro PA, Brown NA, dos Reis TF, Ramalho LN, Savoldi M, Goldman MH, Goldman GH (2012) Molecular characterization of the putative transcription factor SebA involved in virulence in Aspergillus fumigatus. Eukaryot Cell 11:518–531. doi:10.1128/ec.00016-12
Doedt T, Krishnamurthy S, Bockmühl DP, Tebarth B, Stempel C, Russell CL, Brown AJ, Ernst JF (2004) APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol Biol Cell 15:3167–3180. doi:10.1091/10.1091/mbc.E03-11-0782
Dutton JR, Johns S, Miller BL (1997) StuAp is a sequence-specific transcription factor that regulates developmental complexity in Aspergillus nidulans. EMBO J 16:5710–5721. doi:10.1093/emboj/16.18.5710
Ejzykowicz DE, Cunha MM, Rozental S, Solis NV, Gravelat FN, Sheppard DC, Filler SG (2009) The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol Microbiol 72:155–169. doi:10.1111/j.1365-2958.2009.06631.x
Ernst JF (2000) Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology 146(Pt 8):1763–1774. doi:10.1099/00221287-146-8-1763
Errede B, Ammerer G (1989) STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev 3:1349–1361
Ferreira-Nozawa MS, Silveira HC, Ono CJ, Fachin AL, Rossi A, Martinez-Rossi NM (2006) The pH signaling transcription factor PacC mediates the growth of Trichophyton rubrum on human nail in vitro. Med Mycol 44:641–645. doi:10.1080/13693780600876553
Fumeaux J, Mock M, Ninet B, Jan I, Bontems O, Léchenne B, Lew D, Panizzon RG, Jousson O, Monod M (2004) First report of Arthroderma benhamiae in Switzerland. Dermatology 208:244–250. doi:10.1159/000077311
García-Pedrajas MD, Baeza-Montañez L, Gold SE (2010) Regulation of Ustilago maydis dimorphism, sporulation, and pathogenic development by a transcription factor with a highly conserved APSES domain. Mol Plant Microbe Interact 23:211–222. doi:10.1094/mpmi-23-2-0211
Gavrias V, Andrianopoulos A, Gimeno CJ, Timberlake WE (1996) Saccharomyces cerevisiae TEC1 is required for pseudohyphal growth. Mol Microbiol 19:1255–1263
Gimeno CJ, Fink GR (1994) Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol Cell Biol 14:2100–2112
Gräser Y, Scott J, Summerbell R (2008) The new species concept in dermatophytes—a polyphasic approach. Mycopathologia 166:239–256. doi:10.1007/s11046-008-9099-y
Grumbt M, Defaweux V, Mignon B, Monod M, Burmester A, Wöstemeyer J, Staib P (2011a) Targeted gene deletion and in vivo analysis of putative virulence gene function in the pathogenic dermatophyte Arthroderma benhamiae. Eukaryot Cell 10:842–853. doi:10.1128/ec.00273-10
Grumbt M, Monod M, Staib P (2011b) Genetic advances in dermatophytes. FEMS Microbiol Lett 320:79–86. doi:10.1111/j.1574-6968.2011.02276.x
Gu SQ, Li P, Wu M, Hao ZM, Gong XD, Zhang XY, Tian L, Zhang P, Wang Y, Cao ZY, Fan YS, Han JM, Dong JG (2014) StSTE12 is required for the pathogenicity of Setosphaeria turcica by regulating appressorium development and penetration. Microbiol Res 169:817–823. doi:10.1016/j.micres.2014.04.001
Gu Q, Zhang C, Liu X, Ma Z (2015) A transcription factor FgSte12 is required for pathogenicity in Fusarium graminearum. Mol Plant Pathol 16:1–13. doi:10.1111/mpp.12155
Hartwell LH (1980) Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone. J Cell Biol 85:811–822
Hu P, Wang Y, Zhou J, Pan Y, Liu G (2015) AcstuA, which encodes an APSES transcription regulator, is involved in conidiation, cephalosporin biosynthesis and cell wall integrity of Acremonium chrysogenum. Fungal Genet Biol 83:26–40. doi:10.1016/j.fgb.2015.08.003
IpCho SV, Tan KC, Koh G, Gummer J, Oliver RP, Trengove RD, Solomon PS (2010) The transcription factor StuA regulates central carbon metabolism, mycotoxin production, and effector gene expression in the wheat pathogen Stagonospora nodorum. Eukaryot Cell 9:1100–1108. doi:10.1128/ec.00064-10
Li D, Bobrowicz P, Wilkinson HH, Ebbole DJ (2005) A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170:1091–1104. doi:10.1534/genetics.104.036772
Liu H, Köhler J, Fink GR (1994) Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723–1726
Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90:939–949
Madhani HD, Fink GR (1997) Combinatorial control required for the specificity of yeast MAPK signaling. Science 275:1314–1317
Martinez DA, Oliver BG, Gräser Y, Goldberg JM, Li W, Martinez-Rossi NM, Monod M, Shelest E, Barton RC, Birch E, Brakhage AA, Chen Z, Gurr SJ, Heiman D, Heitman J, Kosti I, Rossi A, Saif S, Samalova M, Saunders CW, Shea T, Summerbell RC, Xu J, Young S, Zeng Q, Birren BW, Cuomo CA, White TC (2012) Comparative genome analysis of Trichophyton rubrum and related dermatophytes reveals candidate genes involved in infection. MBio 3:e00259–12. doi:10.1128/mBio.00259-12
Miller KY, Toennis TM, Adams TH, Miller BL (1991) Isolation and transcriptional characterization of a morphological modifier: the Aspergillus nidulans stunted (stuA) gene. Mol Gen Genet 227:285–292
Miller KY, Wu J, Miller BL (1992) StuA is required for cell pattern formation in Aspergillus. Genes Dev 6:1770–1782
Nishimura M, Fukada J, Moriwaki A, Fujikawa T, Ohashi M, Hibi T, Hayashi N (2009) Mstu1, an APSES transcription factor, is required for appressorium-mediated infection in Magnaporthe grisea. Biosci Biotechnol Biochem 73:1779–1786
Nolting N, Pöggeler S (2006) A STE12 homologue of the homothallic ascomycete Sordaria macrospora interacts with the MADS box protein MCM1 and is required for ascosporogenesis. Mol Microbiol 62:853–868. doi:10.1111/j.1365-2958.2006.05415.x
Ohara T, Tsuge T (2004) FoSTUA, encoding a basic helix-loop-helix protein, differentially regulates development of three kinds of asexual spores, macroconidia, microconidia, and chlamydospores, in the fungal plant pathogen Fusarium oxysporum. Eukaryot Cell 3:1412–1422. doi:10.1128/ec.3.6.1412-1422.2004
Padhye AA, Young CN, Ajello L (1980) Hair perforation as a diagnostic criterion in the identification of Epidermophyton, Microsporum and Trichophyton species. Pan Am Health Org Sci Publ 396:115–120
Park G, Xue C, Zheng L, Lam S, Xu JR (2002) MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol Plant Microbe Interact 15:183–192. doi:10.1094/mpmi.2002.15.3.183
Park G, Bruno KS, Staiger CJ, Talbot NJ, Xu JR (2004) Independent genetic mechanisms mediate turgor generation and penetration peg formation during plant infection in the rice blast fungus. Mol Microbiol 53:1695–1707. doi:10.1111/j.1365-2958.2004.04220.x
Pasquali M, Spanu F, Scherm B, Balmas V, Hoffmann L, Hammond-Kosack KE, Beyer M, Migheli Q (2013) FcStuA from Fusarium culmorum controls wheat foot and root rot in a toxin dispensable manner. PLoS One 8:e57429. doi:10.1371/journal.pone.0057429
Raubitschek F, Evron R (1963) Experimental invasion of hair by dermatophytes. Arch Dermatol 88:837–845
Rispail N, Di Pietro A (2009) Fusarium oxysporum Ste12 controls invasive growth and virulence downstream of the Fmk1 MAPK cascade. Mol Plant Microbe Interact 22:830–839. doi:10.1094/mpmi-22-7-0830
Schamber A, Leroch M, Diwo J, Mendgen K, Hahn M (2010) The role of mitogen-activated protein (MAP) kinase signalling components and the Ste12 transcription factor in germination and pathogenicity of Botrytis cinerea. Mol Plant Pathol 11:105–119. doi:10.1111/j.1364-3703.2009.00579.x
Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jochl C, Moussa TA, Wang S, Gsaller F, Blatzer M, Werner ER, Niermann WC, Brakhage AA, Haas H (2010) HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog 6:e1001124. doi:10.1371/journal.ppat.1001124
Sheppard DC, Doedt T, Chiang LY, Kim HS, Chen D, Nierman WC, Filler SG (2005) The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol Biol Cell 16:5866–5879. doi:10.1091/mbc.E05-07-0617
Sigl C, Haas H, Specht T, Pfaller K, Kürnsteiner H, Zadra I (2011) Among developmental regulators, StuA but not BrlA is essential for penicillin V production in Penicillium chrysogenum. Appl Environ Microbiol 77:972–982. doi:10.1128/aem.01557-10
Sonneborn A, Bockmühl DP, Ernst JF (1999) Chlamydospore formation in Candida albicans requires the Efg1p morphogenetic regulator. Infect Immun 67:5514–5517
Soyer JL, Hamiot A, Ollivier B, Balesdent MH, Rouxel T, Fudal I (2015) The APSES transcription factor LmStuA is required for sporulation, pathogenic development and effector gene expression in Leptosphaeria maculans. Mol Plant Pathol 16:1000–1005. doi:10.1111/mpp.12249
Staib P, Kretschmar M, Nichterlein T, Hof H, Morschhauser J (2002) Transcriptional regulators Cph1p and Efg1p mediate activation of the Candida albicans virulence gene SAP5 during infection. Infect Immun 70:921–927
Staib P, Zaugg C, Mignon B, Weber J, Grumbt M, Pradervand S, Harshman K, Monod M (2010) Differential gene expression in the pathogenic dermatophyte Arthroderma benhamiae in vitro versus during infection. Microbiology 156:884–895. doi:10.1099/mic.0.033464-0
Stoldt VR, Sonneborn A, Leuker CE, Ernst JF (1997) Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J 16:1982–1991. doi:10.1093/emboj/16.8.1982
Symoens F, Jousson O, Planard C, Fratti M, Staib P, Mignon B, Monod M (2011) Molecular analysis and mating behaviour of the Trichophyton mentagrophytes species complex. Int J Med Microbiol 301:260–266. doi:10.1016/j.ijmm.2010.06.001
Symoens F, Jousson O, Packeu A, Fratti M, Staib P, Mignon B, Monod M (2013) The dermatophyte species Arthroderma benhamiae: intraspecies variability and mating behaviour. J Med Microbiol 62:377–385. doi:10.1099/jmm.0.053223-0
Tong X, Zhang X, Plummer KM, Stowell KM, Sullivan PA, Farley PC (2007) GcSTUA, an APSES transcription factor, is required for generation of appressorial turgor pressure and full pathogenicity of Glomerella cingulata. Mol Plant Microbe Interact 20:1102–1111. doi:10.1094/mpmi-20-9-1102
Tsuji G, Fujii S, Tsuge S, Shiraishi T, Kubo Y (2003) The Colletotrichum lagenariu Ste12-like gene CST1 is essential for appressorium penetration. Mol Plant Microbe Interact 16:315–325. doi:10.1094/mpmi.2003.16.4.315
Vallim MA, Miller KY, Miller BL (2000) Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol Microbiol 36:290–301
Ward MP, Gimeno CJ, Fink GR, Garrett S (1995) SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol Cell Biol 15:6854–6863
Weitzman I, Summerbell RC (1995) The dermatophytes. Clin Microbiol Rev 8:240–259
White TC, Oliver BG, Gräser Y, Henn MR (2008) Generating and testing molecular hypotheses in the dermatophytes. Eukaryot Cell 7:1238–1245. doi:10.1128/ec.00100-08
Wong Sak Hoi J, Dumas B (2010) Ste12 and Ste12-like proteins, fungal transcription factors regulating development and pathogenicity. Eukaryot Cell 9:480–485. doi:10.1128/ec.00333-09
Wong Sak Hoi J, Herbert C, Bacha N, O’Connell R, Lafitte C, Borderies G, Rossignol M, Rougé P, Dumas B (2007) Regulation and role of a STE12-like transcription factor from the plant pathogen Colletotrichum lindemuthianum. Mol Microbiol 64:68–82. doi:10.1111/j.1365-2958.2007.05639.x
Wu J, Miller BL (1997) Aspergillus asexual reproduction and sexual reproduction are differentially affected by transcriptional and translational mechanisms regulating stunted gene expression. Mol Cell Biol 17:6191–6201
Young LY, Lorenz MC, Heitman J (2000) A STE12 homolog is required for mating but dispensable for filamentation in Candida lusitaniae. Genetics 155:17–29
Zaugg C, Monod M, Weber J, Harshman K, Pradervand S, Thomas J, Bueno M, Giddey K, Staib P (2009) Gene expression profiling in the human pathogenic dermatophyte Trichophyton rubrum during growth on proteins. Eukaryot Cell 8:241–250. doi:10.1128/ec.00208-08
Acknowledgments
This work was supported by the DFG funded excellence graduate school Jena School for Microbial Communication (JSMC; GSC 214; www.jsmc.uni-jena.de) and the Leibniz Institute for Natural Product Research and Infection Biology (HKI) (www.leibniz-hki.de).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study did not include any diagnostic procedure or therapeutic method. Furthermore, the sample collection was non-invasive (the physical integrity of the donor was maintained) and did not intrude into the privacy of the donor. Based on the regulations of the ethics commission at the Jena University Hospital, Jena (Germany), an approval of the study was not necessary in this case.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by M. Kupiec.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1: Primers used in this study
294_2016_608_MOESM1_ESM.docx
Supplementary Fig. S1: Multiple sequence alignments of StuA homologs of N. crassa (Asm-1, XP_960837.1), A. nidulans (StuA, AN5836), A. benhamiae (AbenStuA, ARB_07703); C. albicans (Efg1, CR_07890W_A) and S. cerevisiae (Phd1, CAA81878.1; Sok2, AAB35749.1) using EBI Clustal Omega. The APSES domain is shaded in grey (DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Kröber, A., Etzrodt, S., Bach, M. et al. The transcriptional regulators SteA and StuA contribute to keratin degradation and sexual reproduction of the dermatophyte Arthroderma benhamiae . Curr Genet 63, 103–116 (2017). https://doi.org/10.1007/s00294-016-0608-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-016-0608-0