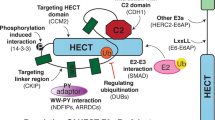
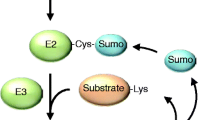
Abstract
Ubiquitin (Ub) regulates numerous cellular processes through covalent attachment to other proteins in the forms of poly- and mono-ubiquitination. A recent study in yeast shows that ubiquitin controls TORC1 through a noncovalent binding with Kog1, a regulatory subunit of TORC1. The binding stabilizes Kog1 and prevents its degradation under stress conditions. This finding unveils a novel role of Ub in TORC1 function and implicates a unique mechanism that attributes the action of Ub in cell signaling.

Similar content being viewed by others
References
Adami A, Garcia-Alvarez B, Arias-Palomo E, Barford D, Llorca O (2007) Structure of TOR and its complex with KOG1. Mol Cell 27:509–516
Grabbe C, Dikic I (2009) Functional roles of ubiquitin-like domain (ULD) and ubiquitin-binding domain (UBD) containing proteins. Chem Rev 109:1481–1494
Heride C, Urbe S, Clague MJ (2014) Ubiquitin code assembly and disassembly. Curr Biol 24:R215–R220
Hicke L, Dunn R (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19:141–172
Ho YH, Gasch AP (2015) Exploiting the yeast stress-activated signaling network to inform on stress biology and disease signaling. Curr Genet 61:503–511
Hochstrasser M (1996) Ubiquitin-dependent protein degradation. Annu Rev Genet 30:405–439
Hoeller D, Hecker CM, Wagner S, Rogov V, Dotsch V, Dikic I (2007) E3-independent monoubiquitination of ubiquitin-binding proteins. Mol Cell 26:891–898
Hu K, Guo S, Yan G, Yuan W, Zheng Y, Jiang Y (2015) Ubiquitin regulates TORC1 in yeast Saccharomyces cerevisiae. Mol Microbiol. doi:10.1111/mmi.13319
Komander D, Rape M (2012) The ubiquitin code. Annu Rev Biochem 81:203–229
Mukhopadhyay D, Riezman H (2007) Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315:201–205
Pashkova N, Gakhar L, Winistorfer SC, Yu L, Ramaswamy S, Piper RC (2010) WD40 repeat propellers define a ubiquitin-binding domain that regulates turnover of F box proteins. Mol Cell 40:433–443
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124:471–484
Yan G, Lai Y, Jiang Y (2012) The TOR complex 1 is a direct target of Rho1 GTPase. Mol Cell 45:743–753
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35
Acknowledgments
The author thanks other laboratory members for critical reading of this manuscript. This study was supported by NIH Grants (CA169186) to YJ. The author has no conflict of interest to declare for this publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Jiang, Y. Regulation of TORC1 by ubiquitin through non-covalent binding. Curr Genet 62, 553–555 (2016). https://doi.org/10.1007/s00294-016-0581-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-016-0581-7