Summary
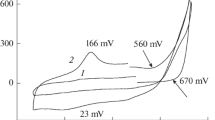
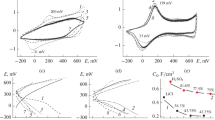
Polyaniline (Pani) was electrogenerated potentiostatically (1 V vs Ag/Ag+) in acid aqueous medium (2 M HNO3) in the presence of LiClO4 on Pt electrodes. The kinetic equation of the polymerization process was calculated by means of the electrochemical data of the polymerization charge (Qpol), determined as Rp= [E]0.14 [M]2. Furthermore, the number of electrons consumed per monomer unit incorporated in the oxidated polymer was determined. The results show that the electrolyte has practically no effect on the reaction kinetics at the concentrations tested, but that it is, moreover, the triggering factor for fewer electrons to be incorporated into the polymer chain.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 17 January 2001/Revised version: 13 May 2001/Accepted: 26 July 2001
Rights and permissions
About this article
Cite this article
del Río, C., Olivares, N. & Acosta, J. Electrogeneration kinetics of polyaniline in the presence of lithium perchlorate. Polymer Bulletin 47, 65–70 (2001). https://doi.org/10.1007/s002890170022
Issue Date:
DOI: https://doi.org/10.1007/s002890170022