Abstract
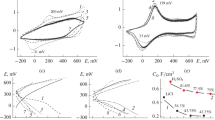
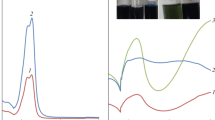
The chemical and electrochemical syntheses of polyaniline (PANI) were performed in a sulfuric electrolyte in the presence of the vanadate anion (VA). The resulting composite materials based on PANI and VA (PANI–VA) were characterized by IR spectroscopy, X-ray diffractometry, SEM, and elemental analysis. In the electrochemical trials, conditions were indicated under which the composite material retained 96% of its electrochemical capacitance (C) and the cycling of PANI–VA in the range of potentials extended to the anodic and cathodic regions did not lead to the degradation of the polymer material over 50 cycles.







Similar content being viewed by others
REFERENCES
Fan, Z., Yan, J., Wei, T., Zhi, L., Ning, G., Liand, T., and Wei, F., Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density, Adv. Funct. Mater., 2011, vol. 21, p. 2366.
Wu, X.-L., Jiang, L.-Y., Cao, F.-F., Guo, Y.-G., and Wan, L.-J., LiFePO4 Nanoparticles embedded in a nanoporous carbon matrix: Superior cathode material for electrochemical energy-storage devices, Adv. Mater., 2009, vol. 21, p. 2710.
Peng, C., Zhang, S., Zhou, X., and Chen, G.Z., Unequalisation of electrode capacitances for enhanced energy capacity in asymmetrical supercapacitors, Environ. Sci., 2010, vol. 3, p. 1499.
Lu, X., Yu, M., Zhai, T., Wang, G., Xie, S., Liu, T., Liang, C., Tong, Y., and Li, Y., High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode, Nano Lett., 2013, vol. 13, p. 2628.
Wang, G., Zhang, L., and Zhang, J., A review of electrode materials for electrochemical supercapacitors, Chem. Soc. Rev., 2012, vol. 41, p. 797.
Yu, G., Hu, L., Vosgueritchian, M., Wang, H., Xie, X., Mc Donough, J.R., Cui, X., Cui, Y., and Bao, Z., Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors, Nano Lett., 2011, vol. 11, p. 2905.
Xiao, J. and Yang, S., Sequential crystallization of sea urchin-like bimetallic (Ni, Co) carbonate hydroxide and its morphology conserved conversion to porous NiCo2O4 spinel for pseudocapacitors, RSC Adv., 2011, vol. 1, p. 588.
Wang, G., Lu, X., Lingm, Y., Zhai, T., Wang, H., Tong, Y., and Li, Y., LiCl/PVA gel electrolyte stabilizes vanadium oxide nanowire electrodes for pseudocapacitors, ACS Nano, 2012, vol. 11, p. 10296.
Wu, C., Feng, F., and Xie, Y., Design of vanadium oxide structures with controllable electrical properties for energy applications, Chem. Soc. Rev., 2013, vol. 42, p. 5157.
Zhu, J., Cao, L., Wu, Y., Gong, Y., Liu, Z., Hoster, H.E., Zhang, Y., Zhang, S., Yang, S., Yan, Q., Ajayan, P.M., and Vajtai, R., Building 3D structures of vanadium pentoxide nanosheets and application as electrodes in supercapacitors, Nano Lett., 2013, vol. 13, p. 5408.
Pletnev, R.N., Gubanov, V.A., and Fotiev, A.A., YaMR v oksidnykh soedineniyakh vanadiya (NMR of Vanadium Oxide Compounds), Moscow: Nauka, 1979.
Mak, W.F., Wee, G., Aravindan, V., Gupta, N., Mhaisalkarand, S.G., and Madhavi, S., High-energy density asymmetric supercapacitor based on electrospun vanadium pentoxide and polyaniline nanofibers in aqueous electrolyte, J. Electrochem. Soc., 2012, vol. 159, p. A1481.
Qu, Q., Zhu, Y., Gao, X., and Wu, Y., Core–shell structure of polypyrrole grown on V2O5 nanoribbon as high performance anode material for supercapacitors, Adv. Energy Mater., 2012, vol. 2, p. 950.
Fotiev, A.A., Trunov, V.K., and Zhuravlev, V.D., Vanadaty dvukhvalentnykh metallov (Bivalent Metal Vanadates), Moscow: Nauka, 1985.
Pelletier, O., Davidson, P., Bourgaux, C., Coulon, C., Regnault, S., and Livage, J., A Detailed study of the synthesis of aqueous vanadium pentoxide nematic gels, Langmuir, 2000, vol. 16, p. 5295.
Wu, C.G., De Groot, D.C., Marcy, H.O., Schindler, J.L., Kannewurf, C.R., Liu, Y.-J., Hirpo, W., and Kanatzidis, M.G., Redox intercalative polymerization of aniline in V2O5 xerogel. The postintercalative intralamellar polymer growth in polyaniline/metal oxide nanocomposites is facilitated by molecular oxygen, Chem. Mater., 1996, vol. 8, p. 1992.
Ferreira, M., Huguenin, F., Zucolotto, V., Pereira da Silva, J. Ed., Cordoba de Torresi, S.I., Temperini, M.L.A., Torresi, R.M., and Oliveira, Os.N., Electroactive Multilayer Films of Polyaniline and Vanadium Pentoxide, J. Phys. Chem. B, 2003, vol. 107, p. 8351.
Hao, Q., Lei, W., Xia, X., Yan, Zh., Yang, X., Lu, L., and Wang, X., Exchange of counterions in electropolymerized PANI film, Electrochim. Acta, 2010, vol. 55, p. 632.
Bai, M.-H., Liu, T.-Yu., Luan, F., Liand, Ya., and Liu, X.-X., Electrodeposition of vanadium oxide–polyaniline composite nanowire electrodes for high energy density supercapacitors, J. Mater. Chem. A, 2014, vol. 2, p. 10882.
Ragupathy, D., Gopalan, A.I., Lee, K.-P., and Manesh, K.M., Electro-assisted fabrication of layer-by-layer assembled poly(2,5-dimethoxyaniline)/phosphotungstic acid modified electrode and electrocatalytic oxidation of ascorbic acid, Electrochem. Commun., 2008, vol. 10, p. 527.
Trchova M., Moravkova Z., Blaha, M., and Stejskal, Ja., Raman spectroscopy of polyaniline and oligoaniline thin films, Electrochim. Acta, 2014, vol. 122, p. 28.
Huguenin, F., Ticianelli, E.A., and Torresi, R.M., XANES study of polyaniline–V2O5 and sulfonated polyaniline–V2O5 nanocomposites, Electrochim. Acta, 2002, vol. 47, p. 3179.
Shouji, E. and Buttry, D.A., New organic-inorganic nanocomposite materials for energy storage applications, Langmuir, 1999, vol. 15, p. 669.
Huguenin, F., Prado Gambardella, M.T., Torresi, R.M., Cordoba Torresi, S.I., and Buttryc, D.A., Chemical and electrochemical characterization of a novel nanocomposite formed from V2O5 and poly(N-propane sulfonic acid aniline), a self-doped polyaniline, J. Electrochem. Soc., 2000, vol. 47, p. 2437.
Geniès, E.M., Lapkowski, M., and Penneau, J.F., Cyclic voltammetry of polyaniline: interpretation of the middle peak, J. Electroanal. Chem. Interfacial Electrochem., 1988, vol. 249, p. 97.
Kieffel, Y., Pierre Travers, J., Ermolieff, A., and Rouchon, D., Thermal aging of undoped polyaniline: Effect of chemical degradation on electrical properties, J. Appl. Polymer Sci., 2002, vol. 86, p. 395.
ACKNOWLEDGMENTS
We are grateful to A.M. Kolesnikova for consultations and technical assistance with this work.
Funding
This study was performed under state assignment (state ID nos. 0089-2019-0010 and 0089-2014-0022, Institute of Problems of Chemical Physics, Russian Academy of Sciences).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Abalyaeva, V.V., Dremova, N.N. Electrochemical Behavior of Polyaniline in the Presence of the Vanadate Anion. Russ J Electrochem 55, 850–859 (2019). https://doi.org/10.1134/S1023193519090027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519090027