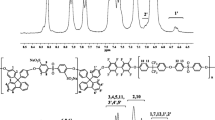
Abstract
The design and development of cost-effective and increased methanol permeable and proton-conductive membranes are critical concerns in the fabrication of polymeric electrolyte membranes (PEM). A solution-casting process was used to create a low-cost PEM based on sulfonated polyvinyl chloride (SPVC)-Polymethyl methacrylate (PMMA) blended with varying concentrations of Poly(sodium 4-styrenesulfonate) (PSS). The contact angle, oxidative stability, swelling ratio, water uptake, and methanol uptake of SPVC/PMMA/PSS membranes were investigated as a function of PSS molar ratio. FT-IR examination, 1H NMR spectra, Raman spectroscopy, X-ray diffraction, thermogravimetric analysis (TGA), and scanning electron microscope micrographs were additionally utilized for confirming the chemical structure, morphology, and thermal stability of SPVC/PMMA/PSS membranes. Furthermore, the ion exchange capacity (IEC), proton conductivity, and methanol permeability of SPVC/PMMA/PSS membranes were investigated depending on the PSS concentration. The results showed a significant increase in proton conductivity from 1.80 × 10–2 for SPVC/PMMA/1%PSS to 4.7 × 10–2 S/cm for SPVC/PMMA/5%PSS at ambient temperature. On the other hand, the methanol permeability (P = 8.53 × 10–8 cm2/s) was noticeably lower than that of Nafion 117 (3.39 × 10–6 cm2/s). Additionally, the IEC of the manufactured membrane was 1.38 ± 0.7 meq g−1 for SPVC/PMMA/5%PSS compared to 0.91 meq g−1 for Nafion 117 membranes. The maximum water uptake of the synthesized membranes was 48.37 ± 2.27%, whereas Nafion 117 membrane absorption was 65.44%. According to conductivity studies and the membrane efficiency factor, the ideal PSS content in a polymer matrix is 4 wt.%. Finally, the developed SPVC/PMMA/PSS polyelectrolytic membranes show improvements in essential properties such as methanol permeability, proton conductivity, and IEC when combined with low-cost materials, making them an attractive contender as PEM for DMFCs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Our everyday activities require the utilization of energy. A quick increase in overall population and constant personal income growth are two factors that contribute to rising energy consumption. By 2035, the global population is expected to reach more than 8.7 billion, implying that another 1.6 billion people will require energy [1]. The fundamental dilemma includes increasing energy use and dwindling fossil fuel availability, as well as concerns about the impact of traditional fossil fuels on human health [2]. Sustainable energy and environmentally friendly alternatives must be used effectively to replace non-sustainable fossil fuels. Fuel cell technology is an innovative technology that provides an immediate resolution to the aforementioned problems and is seen as an interesting alternative to conventional electrical power sources [3, 4].
Direct methanol fuel cells (DMFCs) have recently emerged as the most reliable fuel cells, particularly for portable power applications, due to their high energy transformation effectiveness, low temperatures, environmentally friendly design, high power density, and use of transportable and less expensive fuel. However, one notable restriction of DMFC is the loss of methanol over the polyelectrolytic membrane, which causes extremely reactive polymeric electrolytes with well-organized water management. Furthermore, fouling can affect the efficacy of polymeric membranes by obstructing proton-binding locations, mainly when produced by fuel impurities, other biological products, or chemical processes. The contaminated polymeric membrane must be recovered or replaced to improve operational efficiency. Furthermore, one of the primary hurdles to fuel cell progress is the high cost of components, such as electrodes and electrolytes [5]. The proton exchange membrane (PEM) is a crucial component of DMFCs, which works as both a conductor to move protons (H+) from the anode to the cathode when the fuel and oxidizer are separated in the chambers and functions as a barrier to prevent methanol from penetrating [6,7,8].
A compatible PEM must demonstrate high conductivity, significant water absorption capacity, low permeability to the fuel, exceptional mechanical and oxidative stability, electricity-insulating properties, and the ability to be developed as a membrane electrode assembly (MEA). With all the characteristics mentioned above, it is critical that the commercial sector maintains as low pricing as possible. The primary problem is to create a low-cost alternative membrane with high performance [9]. Recently, Nafion®, a perfluorinated sulfonic acid (PFSA) ionomer-based membrane, is the most commonly used membrane for electrolytic membranes due to its unique hydrophilic/hydrophobic nanophase structure, substantial proton conductivity, high thermal stability, good mechanical, and chemical properties at room temperature. However, Nafion® is difficult to synthesize and handle [10]. In the present situation, methanol crossover is the passage of methanol from the anode to the cathode side of a fuel cell via the membrane. Furthermore, the polyelectrolyte membrane (PEM)’s high methanol permeability causes fuel loss and decreased fuel cell efficiency.
To overcome these challenges, considerable research has been conducted over the last three decades to create innovative polyelectrolyte membranes as substitutes for Nafion®. As Nafion® membrane substitutes, hydrocarbon-based PEMs have been the focus of comprehensive studies due to their economical price, ideal molecular structure, enhanced film-forming capability, manufacturing flexibility, and mechanical and thermal properties. Moreover, these compounds offer irregular sites for the electrophilic substitution of SO3H groups, permitting extraordinarily high proton conduction. Notably, as practical hydrocarbon membranes, the sulfonated derivatives of Polybenzimidazoles, Poly(arylene ether sulfones), Polyimides, Polyether ketone, and Polyether ether ketone have attracted much attention lately. Hydrophilic groups in hydrocarbon polymers produce simple swelling or instant hydrogel formation, which is the fundamental obstacle [11].
A wide range of different natural and synthetic polymers have undergone chemical modification or have been grafted with acidic groups, commonly sulfonic groups. Additionally, polymer blending or composite formation involving inorganic compound nanoparticles has taken place. The development of polyelectrolyte membranes has drawn a lot of attention to a variety of synthetic polymers, the most prominent of which is polyvinyl chloride (PVC), an inexpensive and eco-friendly polymer with superior film-forming capabilities, chemical resistance, mechanical strength, and thermal stability [12]. Polyvinyl chloride (PVC) is employed in various industries, construction materials, and biomedical uses.
In solid-state lithium batteries, PVC/lithium sulfate (Li2SO4) has been used as a solid polymer electrolyte (SPE) despite PVC’s superior mechanical properties, chemical durability, and affordability (a 10-by-15-cm Nafion sheet costs $65, compared to $1.34 for a 4-inch PVC sheet) [13]. The use of PVC in PEMs for fuel cell applications has drawn some scientific attention. In one of these unconventional studies, Polystyrene (PS) was synthesized throughout the pores of a PVC membrane to create sulfonated polystyrene (SPS)/PVC composite membranes. In a separate research, PVC membranes that had undergone chemical alteration using ethylene diamine were doped using ortho-phosphoric acid (OPA).
In accordance with these studies, we synthesized a blended membrane based on SPVC/PSS-PMMA polyelectrolytic membranes by utilizing the critical benefits of PVC by functionalizing it with a new proton conduction characteristic via a quick and easy sulfonation technique [14]. Additionally, by mixing it with other substances, it acts as conductive material that includes sulfonic acid groups, such as poly (sodium 4-Styrene sulfonate) (PSS) in different molar ratios; the resultant sulfonated polymer’s proton conduction has been improved [15,16,17]. Incorporating PMMA into SPVC/PSS-based membranes provides reinforcement, creates a meandering channel for methanol to cross over while maintaining water swell-ability, and may help solve the difficulties with methanol crossover and water management in fuel cells. Additionally, including PMMA can increase ionic conductivity by contributing to a significant interfacial surface, ionic channels, and mechanical stability [18]. Optimum conditions were investigated by fabricating a series of blended polymers by adjusting the mixing ratio of PSS in polymer blends to investigate the physicochemical stability, electrochemical characteristics, and structural properties of the developed SPVC/PSS-PMMA polyelectrolytic membranes. The developed membranes’ chemical structures, morphological, and thermal properties were verified by utilizing FT-IR, Raman spectroscopy, 1H NMR spectra, SEM, XRD, and TGA. Furthermore, the contact angle, swelling ratio, water and methanol uptake, tensile strength (TS), ion exchange capacity (IEC), oxidative stability, methanol permeability, and proton conductivity were conducted.
Experimental section
Materials
Polyvinyl chloride (PVC) with high molecular weight (Mw = 48,000 g mol−1) as fine powder was purchased from Honeywell Fluka. Poly (sodium 4-styrene sulfonate) (PSS) (average Mw ~ 70,000) and Methyl Methacrylate (99%) were purchased from Sigma-Aldrich, Germany. Sulfuric acid (Molecular weight ~ 98.08 g mole−1, purity 98%) received from United Company. Tetrahydrofuran (THF) has a purity of 99.9% from PanReac AppliChem (EU). Sodium chloride (NaCl) (purity 99.9%) was purchased from Raheja Center, India. Sodium hydroxide (98%, extra pure pellets) was purchased from Thermo-Scientific Chemicals. Potassium persulfate (KPS) (purity 99%), Methanol absolute (99.5%), and Ethanol absolute (99.8%) from Sigma-Aldrich, Germany. Throughout the study, distilled water was utilized. All other compounds were used without being purified further.
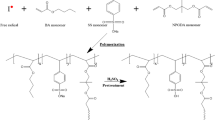
Synthesis of sulfonated polyvinyl chloride (SPVC)
Sulfonated polyvinyl chloride (SPVC) was created using the following technique. To begin, 4 g PVC was dissolved in 20 mL of Tetrahydrofuran (THF) and agitated at 70 °C to achieve dissolution and a homogeneous solution. The obtained solution was then frozen in an ice bath to 0 °C before adding sufficient H2SO4 (5 M) dropwise to the PVC solution. The mixture was then heated for 4 h at 50 °C and allowed to cool. (Scheme 1 illustrates the reaction). After precipitating the mixture using anhydrous ethanol, the residue was washed with anhydrous ethanol until the pH reached 6.0. The resulting SPVC was dried in a vacuum oven at 50 °C for 48 h [19, 20].
Schematic illustration for the synthesis of SPVC by the sulfonation process of PVC [13]
Fabrication of poly(methyl methacrylate) (PMMA)
The polymerization technique, as shown in Scheme 2, was carried out by dissolving 10% (v/v) methyl methacrylate (MMA) monomer in a 50% alcoholic solution containing (0.01 M) potassium persulfate (KPS) as an initiator and heating at 55 °C for 4 h in a shaking water bath. The residue was then rinsed with anhydrous ethanol until it reached pH 6. The resultant PMMA was dried for 48 h in a vacuum oven at 40 °C.
Schematic illustration for the synthesis of Poly(methyl methacrylate) (PMMA) by free radical addition polymerization of methyl methacrylate (MMA) [21]
Preparation of SPVC/PMMA/PSS-based composite polyelectrolytic membranes
To create the targeted polyelectrolyte membranes, a pre-weighed amount of SPVC (4 wt.%) was first dissolved in Tetrahydrofuran (THF) at 70 °C. Then, different concentrations of PSS were added separately to the homogeneous SPVC solutions to achieve functionalization. To make sure that the blend of membrane-forming solutions was sufficiently dissolving, they were continuously agitated for at least one day at room temperature.
The SPVC/PSS blend solutions were subsequently mixed with a pre-weighted amount of PMMA (0.4 wt.%) to increase its mechanical characteristics and thermal stability, yielding the polymeric blends SPVC/PMMA/1%PSS, SPVC/PMMA/2%PSS, SPVC/PMMA/3%PSS, SPVC/PMMA/4%PSS, and SPVC/PMMA/5%PSS. Finally, polyelectrolytic membranes have been created by casting the prepared polymer blend solutions into glass Petri plates and drying them in a vacuum oven at 45 °C for 12 h [22].
Characterization of the synthetic polymeric membranes
Structural properties
The Shimadzu FT-IR-8400 spectrometer, with a scanning rate of 30 s and a resolution of 4 cm−1, was used to analyze the chemical composition of the polymeric membrane components and confirm the fabrication process in the 400–4000 cm−1 range.
The 1H nuclear magnetic resonance (NMR) spectra of the materials were acquired at 25 °C with a Varian (VX-NMR 300 MHz) spectrometer (Bruker, USA). The sample for NMR spectroscopy has been prepared by dissolving approximately 20 mg of the sample in 1 mL of DMSO-D6, and the chemical changes were reported in parts per million (ppm) units using tetramethylsilane (TMS) as the internal standard reference.
Furthermore, a laser Raman scattering spectrometer (SENTERRA-Bruker, Germany) was used in conjunction with a Leica microscope to analyze the chemical interactions and possible connections within the developed polymeric membranes [23, 24].
X‑ray diffractometry (XRD)
The Shimadzu X-ray diffractometer (XRD-6000), which is a measuring instrument with a monochromatic back and a Cu anticathode, was used to conduct structural examinations of the samples. The radical was examined via reflection scanning, with 2 traveling from 0 to 120° [25].
Morphological features and microstructure
Scanning electron microscope (SEM) instrument (JEOL JSM-6360LA, Akishima, Japan) was utilized to investigate the microstructure and morphology of polymer composite membranes by providing high-resolution images of a wide range of membrane surfaces and cross sections. Specimens of the resulting membranes were tested at a 10 kV acceleration voltage. Magnification ranged from 500 to 5000 times [26, 27].
Thermogravimetric analysis (TGA)
The thermal stability of the resulting polymeric materials was assessed using the thermogravimetric technique. A Shimadzu TGA-50, Shimadzu (Kyoto, Japan) thermogravimetric analyzer was used to measure the weight loss of the heated materials. At the same time, the temperature increased steadily at a constant rate (10 °C/min) while being heated with N2 gas. The specimens were heated to temperatures between ambient and 800 °C [28].
Mechanical strength
The synthesized polymeric membranes’ tensile strength and elongation at break were assessed using a Shimadzu universal testing machine (UTM), and the air-dried membranes were first conditioned in a humidity chamber of humidity at 252 °C with 502 °C relative humidity before further mechanical testing. The samples measured 30 × 10 mm. Crosshead observations were made at a constant pace of 5 mm/min [29].
Wettability measurement
By measuring the contact angle of a water droplet with the polymer electrolyte membrane using a sessile drop method (VCA-2500 XE, AST products) connected to a CCD camera and analytic software (AST Products, Billerica, MA, USA), the potential for wettability or hydrophilicity of the produced polymer electrolyte membranes was explored [30, 31].
Ion exchange capacity and sulfonation degree
The IEC of the generated SPVC/PMMA/PSS composite polyelectrolyte membranes has been estimated using acid–base titration approaches. IEC indicates the number of protons donor groups and thus the proportion of PSS in blend-based membranes containing sulfonic groups. Specimens of the same weight were immersed in a 2M sodium chloride solution for 24 h. The protons produced via these samples were measured using acid–base titration utilizing 0.01N sodium hydroxide and phenolphthalein as indicator. The IEC of PEMs was calculated using Eq. (1).
N and V represent the sodium hydroxide solution’s normality and volume titer, respectively, and W signifies the sample weight [32].
The resulting IEC measurements were employed for determining the degree of sulfonation (DS) in accordance with Eq. (2) [13].
where M.(PVC) is the molecular weight of the pure PVC repeating unit, and M(SPVC) is the molecular weight of the SPVC repeating unit (containing –SO3H functional group) [33].
Water uptake, methanol uptake, and swelling properties
The produced polyelectrolytic membranes’ water absorption capacity and swelling ratio (SR) are evaluated by comparing their weights (W) and dimensions (D) in the direction x before (Wdry and Ddry) and after (Wwet and Dwet) immersion in water. The membrane-derived specimen had previously been dried in a vacuum oven at 50 °C for 12 h before estimating its weight and dimensions. The dehydrated polymer membrane samples are immersed in deionized water at room temperature for a full day and then soaked with filter paper to remove surface moisture before weighing the specimen. The water uptake and swelling ratio of the samples are then determined using Eqs. (3, 4).
where Wdry and Ddry represent the mass and dimension of the dehydrated membrane sample, respectively, and Wwet and Dwet represent the mass and dimension of the fully hydrated membrane sample. The same method used to evaluate water intake was used for assessing methanol uptake, substituting water with methanol [34,35,36].
Oxidative stability analysis
Membrane durability is significantly impacted by chemical degradation. When contaminated metal cations like Fe2+ and Cu2+ mix with hydrogen peroxide (H2O2) produced during fuel cell operation, hydroxyl and hydroperoxyl radicals are created that cause damage and speed up membrane degradation. The Fenton test was used to investigate the oxidation stability of manufactured polyelectrolytic membranes. Oxidative stability served as an indicator of the membrane’s long-term sustainability in certain regards. For one hour at 80 °C, the 1 × 4 cm2 dry membrane specimens were immersed in Fenton’s reagent (2 ppm FeSO4 in 3% H2O2). Equation (5) can be used to calculate the oxidative stability based on the weight differential between the samples before and after soaking [35].
where Wo and W1 refer to the weight of the material in grams prior to and following oxidation, respectively.
Methanol permeability measurements
The glass diffusion cell used to measure the permeability of methanol is shown in Fig. 1. It is composed of two glass sections (chamber A, which has a capacity of 125 ml for the feed, and chamber B, which has a capacity of 120 ml for the permeation), divided by a specimen that has the same diameter as the tested polyelectrolytic membrane. Compartment A hosted 15% methanol, whereas Compartment B hosted deionized water. To evaluate the methanol permeability, 1 ml liquid samples from the permeate were taken, while both solutions were kept stirring. A portable density meter (DMA 35-Anton Paar apparatus) was utilized to ascertain the amount of methanol present in liquid samples. Equation (6) was used to calculate the methanol permeability (P) of the prepared membranes [37, 38].
where VB is the initial volume of deionized water, CA is the initial methanol concentration in the compartment (A), K is the permeation curve slope, L is the membrane thickness, and A is the membrane working area (area of permeation).
Methanol permeability via PEMs was measured using two-chamber diffusion cells [39]
Electrochemical measurement
The proton conductivity of the developed polyelectrolyte membranes was investigated using a four-point collinear probe technique. Electrochemical impedance spectroscopy (EIS) using a Gamry Instruments Reference in galvanostatic mode with an AC amplitude of 0.1 mA at frequencies ranging from 1 MHz to 10 Hz was used to assess the ionic conductivity on a palletized sample. A Bode plot was used to investigate the frequency range throughout which the impedance had a constant value, and a Nyquist plot was used to determine the electrical resistance. Equation (7) was utilized to determine the ionic conductivity (σ) [40].
where W is the membrane width (2 cm), T is the membrane thickness, L is the distance between potential-sensing electrodes (1 cm), and R is the synthesized membrane resistance. For conductivity evaluation, the membrane was immersed in a (1 M) sulfuric acid solution at room temperature for 4 h. The membrane was washed many times with deionized water to remove any leftover sulfuric acid. Before conductivity testing, all membranes had been placed in deionized water at room temperature.
Membrane efficiency
To evaluate the suitability of the developed polyelectrolyte composite membranes for use in DMFCs, the membrane efficiency, which expresses proton conductance and methanol permeability, can be computed using Eq. (8) [41].
where membrane’s efficiency is represented by (Φ), which depends on the relationship between proton conductivity (σ) and methanol permeability (P).
Results and discussion
FTIR spectral analysis
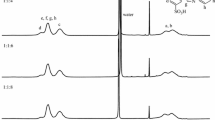
FTIR spectroscopy is used to analyze the chemical structure of composite membranes as well as possible interactions between the functional groups of polymer membrane components. Figure 2 shows the FT-IR spectra of the pristine SPVC and SPVC/PMMA/PSS composite polyelectrolyte membranes. FTIR spectra of pristine SPVC showed a distinctive adsorption peak at 642 cm−1 that was brought on by the stretching of C–Cl. On the other hand, the C–C and C-H adsorption peaks appeared at 1384 and 2939 cm−1, respectively. An absorption band was observed at 1219 cm−1. These bands are made up of sulfonic acid groups (–SO3H), which connect S=O stretches to polymer chain adhesion [42]. On the other hand, the FT-IR spectra of SPVC/PMMA/PSS composite membranes illustrate several peaks, including an adsorption peak at 630 cm−1 produced by C–Cl stretching, two strong, distinct peaks at 1062 and 1220 cm−1, and others [43]. Changes in the vibrational frequency of the peaks and enhanced amorphous phase in the SPVC/PMMA/PSS system demonstrate blend polymer compatibility. Additionally, the strong peaks in absorption bands at roughly 1715 cm−1 and 2939 cm−1 are attributed to the vibration of the C=O of the PMMA component in the polymer blend. The absorption band at around 1435 cm−1 is attributed to the symmetrical stretching vibration of the (–C–O–C–) functional group [44]. The symmetrical O=S=O stretching vibration and the asymmetric stretching vibration of the sulfonic groups were associated with the absorption peak at 1080 cm−1 and 1219 cm−1, respectively. However, due to a rise in SO3H groups, the intensity of these adsorption peaks grew as PSS concentration rose. Additionally, prolonging OH led to the appearance of an adsorption band at 3500 cm−1. On the other hand, when the quantity of sulfonic groups grows, the strength of the OH peak does as well [45, 46].
1H-NMR spectra
The chemical structure of pure PVC and SPVC polymers was characterized by 1H-NMR, and these results are presented in Fig. 3. The 1H NMR of pure PVC displayed chemical shifts at δ = 2.25 ppm and δ = 4.2 ppm, corresponding to the protons of methylene (–CHCl–CH2–) and the methine (–CHCl–CH2–) groups, respectively [47,48,49]. The identical peaks of pure PVC have also originated in the 1H NMR spectrum of SPVC after the sulfonation procedure [δ = 2.25 ppm (–CHCl–CH2–), δ = 4.3 ppm (–CHCl–CH2–)]. In addition, the presence of new chemical shifts at δ = 2.4 ppm in the 1H NMR spectrum of SPVC demonstrates its fabrication [50,51,52]. These chemical shifts are linked to the sulfonic acid protons (–CH2–CH–SO3H) confirming the successful sulfonation process of PVC and preserving the majority of polymer bonds without disintegration.
Raman scattering spectra
Figure 4 displays the Raman scattering spectrum of synthetic SPVC and SPVC/PMMA/PSS composite polyelectrolyte membranes. A significant valence band at 620 cm−1 was visible in the range of a pure SPVC membrane because of the C–Cl bond. As a result, additional signals at 1189 cm−1 and 1456 cm−1, respectively, correspond to the vibration of C–C and C–H bonds. Stretching –SO3H group from the sulfonation process of Pure PVC causes characteristic scattering peaks at 1107 and 1041 cm−1. In contrast, the stretch of carbonyl group (C=O), on the other hand, corresponded to a distinct scattering peak in the Raman spectra of the SPVC/PMMA/PSS composite membranes at around 1696–1725 cm−1. The intensity of this band increases as the PMMA and SPVC/PSS blend are functionalized and chemically cross-linked [53]. At 1041 cm−1, the distinctive peak related to the stretching sulfonic acid group (–SO3H) developed. However, the strength of this peak rose as the proportion of PSS in the polymer mix increased. This rise is related to an increase in the –SO3H group in the composite polyelectrolyte membranes of SPVC/PMMA/PSS. The vibrating of C–H bonds produced spectral signals at 1453 cm−1 [54]. Because the manufacturing and functionalization processes had no major effect on the structure of SPVC, PSS, or PMMA, it is worth noting that all scattering spectra in Fig. 4 follow the same pattern and are relatively comparable.
Morphological and microstructure features
Using a scanning electron microscope (SEM), the surface and cross-section morphological characteristics of pristine SPVC and SPVC/PMMA/PSS composite polyelectrolytic membranes have been examined, as shown in Figs. 5 and 6, respectively. SEM micrograph of the surface of a pristine SPVC-based membrane is shown in Fig. 5(a), which illustrates a thick, dense, and pore-like structure membrane. This might decrease the SPVC polymer solution’s thermodynamic stability, which could significantly accelerate membrane formation by allowing for phase inversion. The possible explanation is that the high viscosity of the SPVC solution physically prevents solvent and non-solvent mass transfer, slowing and preventing the formation of macro-voids in the membrane [55]. The morphological characteristics of the surface of SPVC/PMMA/PSS composite polyelectrolyte membranes with different PSS loadings are displayed in Fig. 5(b–f). These microscopy images showed that these membranes are rougher on the surface and more homogeneous and compact than pristine SPVC-based membranes. While the resulting polyelectrolytic membranes had a more homogeneous surface and less porosity when PSS was incorporated into the polymer matrix, they also had a more compact and rigid framework and did not result in the creation of loose structures or wrinkling fractures in blend membrane matrices.
The inclusion of PSS and PMMA on the SPVC molecular chain was the cause of the casting solution’s comparatively higher viscosity as well as easier gelation. As a result, cross-sectional micrographs of the modified membranes revealed a denser and more compact structure when compared to the pristine SPVC-based membrane as shown in Fig. 6. After the inclusion of PSS, the membrane surface becomes increasingly hydrophilic, and the level of attraction between the membrane and water rises, causing the polymeric membrane to grab more water and the diffusion of water into the polymer matrix to speed up. This leads to reduced methanol crossover and improved swelling properties of membranes, resulting in membrane conductivity. Additionally, as the molar ratio of PSS increases, more sulfonic acid groups (-SO3H) are inserted, which promotes polymer chain tying and alignment. To summarize, all developed SPVC/PSS-PMAA-based polyelectrolyte membranes showed looser and denser microstructures compared to the virgin SPVC-based membrane. These results are supported by hydrogen bonds between polymer chains containing hydrophilic groups.
These findings could explain the exceptional absorption and mix compatibility of membrane blend components that exhibit no phase separation or breaking. The variations seen in the topographical surface and cross section are due to sulfonation sorting, which significantly affects the membrane’s property and structure. It is critical to recognize that the visible cells and holes in the pure SPVC-based membrane are drying patterns. Strong interactions between SPVC/PSS and PMMA chains, enhanced by the hydrogen bonding of their sulfonic groups, enabled the creation of mixed membranes with comparable morphologies. It is worth noting that surface pores developed on the surface of micrographs but did not extend across the membrane, which may have resulted in the smooth transport of protons through the membrane and prevented methanol from crossing [56, 57].
X‑ray diffraction (XRD) patterns
XRD techniques are valuable nondestructive characterization tools for analyzing the crystalline nature and phase purity of synthesized polyelectrolytic membranes.
The X‑ray diffraction patterns of pure SPVC and composite SPVC/PMMA/PSS polyelectrolyte membranes are shown in Fig. 7. When a noticeable peak at around (2θ = 23°) was found, the amorphous phase of pristine SPVC was recognized, which is related to the (002) plane hexagonal lattice.
The exceptionally sharp peaks in the polymer structure can be attributed to the increased crystallites caused by hydrogen bonding between sulfonic groups, which facilitates polymer chain alignment. The results of XRD revealed that the resultant SPVC/PMMA/PSS composite membranes with various PSS loadings have the same diffraction peaks as pristine SPVC. However, increasing the quantity of PSS in the mixed membranes lowered the crystalline regions of SPVC. Moreover, interactions between components that constitute the membrane might be responsible for these results by preventing chains of polymers from accumulating [58].
Thermal stability analysis
Since thermal stability significantly affects the polyelectrolyte membrane’s working life in fuel cell applications, thermogravimetric estimations of the Pure PVC, SPVC, and SPVC/PMMA/PSS composite polyelectrolyte membranes with varying PSS loadings have been determined as illustrated in Fig. 8. These estimates show the weight loss percentage as a function of temperature.
As can be seen, the pristine PVC and SPVC thermograms showed that the weight loss of pristine PVC was (16%) at ~ 260 °C of its starting weight, whereas it was (20%) for SPVC at the same temperature. This was probably attributable to the decomposition of C–Cl bonds in longer polymer chains, and at the beginning of this phase, the physically linked solvent is volatile. While the pristine PVC lost just (42%) of its starting weight at ~ 400 °C, SPVC lost around (64%) of its initial weight at the same temperature, possibly due to C-H bond breaking and backbone fragmentation. At ~ 800 °C, SPVC loses about ~ 98% of its starting weight due to the disintegration of the polymer main chain, whereas pristine PVC loses just ~ 70% of its starting weight at the same temperature. Furthermore, the decomposition of additional suspended groups, which are terminal polymer groups and sulfonic acid groups, which belonged to SPVC, occurs within a temperature range of roughly 200–350 °C [59]. The basic polymer chains are more probable to break at higher temperatures.
The thermograms of the developed polyelectrolytic membrane based on SPVC/PMMA/PSS reveal three regions of weight loss. The first region can be detected between 50 and 210 °C and causes a weight loss of 18%, in which the physically bonded solvent is volatile at the beginning of this region. The second stage of degradation, starting at ~ 220 °C and ending at 350 °C , results in a weight loss of 65% due to C-H bond breaking and backbone fragmentation. The third stage of deterioration, starting at ~ 350 °C and ending at 600 °C causes a 99% weight loss due to the disintegration of the polymer main chain. TGA curves of the synthesized polyelectrolytic membranes also exhibited multi-step weight loss events, with new steps occurring in a range of 200–350 °C can be ascribed to the decomposition of sulfonic acid (–SO3H) groups by de-sulfonation [60].
Surprisingly, the developed composite polyelectrolyte membranes behave almost precisely as pristine SPVC membranes until about 240 °C; following that, the composite membranes act differently from SPVC. This variation can be attributed to the fact that sulfonic acid (–SO3H) groups degrade at lower temperatures in comparison with the polymer matrix main chain. It is possible that the different distribution of sulfonic acid groups inside the chains is the reason why not all sulfonic acid groups break down at the same temperature [61, 62]. As a result, weight loss (%) increases in direct proportion to the degree of sulfonation or the molar concentration of PSS (–SO3H groups) [13]. Nonetheless, the thermal analysis results showed that the developed polyelectrolytic membranes have very considerable thermal stability and are supposed to stay stable enough over the possible temperature range for PEMFC application.
Water uptake, methanol uptake, and swelling properties
Water uptake and swelling ratio are critical variables for improving fuel cell performance by enhancing conductivity because the amount of water in a polyelectrolyte membrane has a substantial influence on proton conductivity and, therefore, fuel cell efficiency. To reduce fuel cross-over in DMFCs using methanol as a hydrogen feedstock, the polyelectrolyte membrane must absorb less methanol. As a result, the water uptake, methanol uptake, and swelling ratio for the constructed pristine SPVC and SPVC/PMMA/PSS composite polyelectrolyte membranes were assessed for their capacity to absorb water and methanol, and the findings are shown in Table 1.
These results demonstrate that blending SPVC with PSS increases the water-holding capacity of the resulting composite-based PEMs. Furthermore, when the fraction of PSS in the blends increased, correspondingly increased the percentage of water absorption. The uptake of water was lower in SPVC/PMMA/PSS-based polyelectrolyte membranes prepared at a ratio of 1%wt.% PSS than in those created at 5 wt.% PSS. The most understandable discussion is that as the proportion of conducting material (PSS) increases, so does the availability of hydrophilic groups (–SO3H), hence increasing the water sorption capacity of polyelectrolyte membranes overall. The investigation demonstrates that PSS has a considerable effect on the polymer matrix, improving the ability of the produced membrane to retain water.
The ability of a polymer membrane to uptake methanol is greatly influenced by its affinity and the pores in the membrane’s structure that allow for liquid absorption. Table 1 shows that synthesized polyelectrolytic membranes with higher water-holding capacity absorbed less methanol. Furthermore, these data demonstrated that when the PSS molar ratio increased the percentage of methanol absorption decreased. The methanol uptake values reached 8.33 ± 1.20% for 1 wt.% PSS-based membranes and 3.63 ± 0.50% for 5 wt.% PSS-based membranes, respectively. The findings have shown that the modification method of SPVC/PMMA/PSS membranes has a more significant impact on decreasing the methanol holding capacity of the manufactured membranes than the non-modified SPVC membrane, hence reducing methanol crossover. On the contrary, the produced polyelectrolytic membranes with higher water-holding capacity showed lesser methanol uptake. This observation can be explained to the hydrophilic and hydrophobic behavior of polymer matrixes [63, 64].
The bulky hyperbranched structure of the developed polyelectrolytic membranes, as well as the relatively strong electrostatic contacts and H-bonding between the blending materials, resulted in an appropriate swelling ratio.
The swelling ratio of developed polyelectrolyte membranes results from an equilibrium of osmotic and other dispersive forces. The dispersion force exerted by the polymer chain balances the osmotic force that attracts water into the polymer matrix. The modified SPVC/PMMA/PSS blend membranes exhibited higher swelling values and may store more water inside the polymer matrix than the pristine SPVA membranes. Swelling variations in these membranes are directly proportional to water absorption and IEC, and enhanced swelling properties of polyelectrolytic membranes have been corresponding to a more hydrophilic membrane surface, which is induced by high-density hydrophilic groups (-SO3H) in PSS and SPVC, reaching a maximum value of 31.74 ± 0.15% for SPVC/PMMA/5%PSS. As a result, all modified polyelectrolytic membranes have a satisfactory swelling ratio, as excessive swelling might cause membranes to dissolve in water, which is undesirable for membranes used in PEMFCs.
While the cathode portion of the fuel cell emits water, the membrane is always in contact with it. As a result, the fuel cell membrane needs to be durable in water. The membrane loses functionality as it dissolves in water. Membranes should not be soluble in water; if they are, their solubility should be kept as low as possible [35].
Mechanical characterization of PEMs
The mechanical characteristics of PEMs considerably influence the consistency of fuel cell performance [65]. Efficiency is a crucial factor in any system, and when it comes to membrane performance, mechanical qualities play a vital role. Two key metrics used to evaluate a membrane’s mechanical properties are elongation and tensile strength. Elongation measures the membrane’s ability to stretch, while tensile strength represents the maximum stress the membrane can withstand during a tensile test. In Table 2, we can see that the tensile stress was increasing as the PSS percentage increased due to high crosslinking process till the highest stress was recorded for SPVC/PMMA/3%PSS (6.72 ± 0.81N/mm2). The tensile stress was decreased and reached 3.54 ± 0.10 N/mm2 for the SPVC/PMMA/5%PSS membrane, but the membranes can satisfy the requirement of the membrane electrode assembly. In addition, the maximum elongation at break increased after increasing PSS percentage from 17.12 ± 1.27% to 44.77 ± 1.50% for SPVC/PMMA/1%PSS and SPVC/PMMA/4%PSS, respectively. However, the maximum elongation for SPVC/PMMA/4%PSS and SPVC/PMMA/5%PSS membranes decreased from 44.77 ± 1.50% to 20.10 ± 2.20%. These findings suggest that crosslinking membranes have better mechanical properties as increasing PSS percentage till (SPVC/PMMA/4%PSS) membrane and could potentially be more efficient in certain applications. The thermal stability data acquired from the TGA investigation supports this idea [66, 67].
Ion exchange capacity and sulfonation degree
The ion exchange capacity (IEC) of pristine SPVC and SPVC/PMMA/PSS composite PEMs was measured at room temperature. IEC provides a second-hand and valid estimate of proton conductivity because it reveals the concentration of transferable protons (sulfonic groups) traveling across polymer chains in charge of proton transportation [68]. Figure 9 displays the IEC measurements of the abovementioned synthetic membranes. There is no IEC in pure PVC-based membranes since there are no ionizable functional groups. The resulting SPVC membrane has an IEC value of 0.24 ± 0.03 meq g−1 due to the sulfonation method, suggesting the presence of sulfonic acid groups on the polymer backbone. Combining SPVC and PSS will raise the IEC of the composite-based membranes. Consequently, adding PSS and SPVC increases the IEC of the composite-based membranes produced. The highest IEC value (1.38 ± 0.7 meq g−1) was recorded for the SPVC/PMMA/5%PSS blend-based membrane, and the IEC value also increased as the percentage of PSS in the blends increased. Due to a rise in the (i.e., SO3−1) percentage because of SPVC sulfonation and an elevation in the quantity of PSS in the combinations, the IEC for SPVC and SPVC/PMMA/PSS composite-based membranes has increased [69].
The degree of sulfonation (DS) of sulfonated polymer (SPVC) can be estimated from its ion exchange capacity value (0.24 ± 0.03 meq/g), which corresponds to the DS value of 1.55%. Multiple research investigations have demonstrated that the generated polyelectrolyte membranes’ IEC is an important parameter since it controls the physicochemical characteristics of PEM. For example, high IEC is typically required to achieve high proton conductivity. Sadly, high DS values result in inadequate thermal stability, poor mechanical qualities, and significant membrane swelling—all of which are undesirable in fuel cell applications. In summary, for the fuel cell to operate satisfactorily, the membranes must offer sufficient ionic conductivity as well as mechanical, thermal, and hydrolytic stability [13, 70].
Contact angle measurements of PEMs
A PEMs wettability reflects its proclivity to swell or absorb water, which is connected to the produced membranes’ IEC, water stability, and proton conductivity. As a result, this feature was studied using the water contact angle. A smaller contact angle generally suggests a more hydrophilic membrane surface [71]. Figure 10 displays contact angle images using a contact angle meter apparatus, and Fig. 11 displays the findings of measuring the water contact angles of pure SPVC and SPVC/PMMA/PSS-based polyelectrolyte membranes to ascertain whether the membrane surface was hydrophilic or hydrophobic. It can be revealed that the water contact angle (WCA) of the pristine SPVC-based membrane is 67.9°, which is lower than the Nafion 117 membrane, which is 110°, and this is due to the presence of a more significant number of hydrophilic sulfonic groups (–SO3H) being substituted onto polymer chains during the sulfonation process. Furthermore, combining SPVC/PSS with PMMA to create SPVC/PMMA/PSS composite polyelectrolyte membranes resulted in a lower contact angle overall. As the concentration of PSS in the polymer matrix grew, these decreases increased, implying that the hydrophilicity of SPVC/PMMA/PSS composite membranes increased considerably. Furthermore, water dropped on modified membranes penetrated the membranes quicker than virgin SPVC. Consequently, the membranes based on the SPVC/PMMA/5%PSS blend exhibited the lowest contact angle. These findings show that PSS and SPVC are continually blended while increasing their interface, demonstrating the synergy between these polymer components in reducing surface hydrophobicity [72].
Proton conductivity measurements
Figure 12 displays the proton conductivity data for synthetic polyelectrolytic membranes as a function of PSS loading. The virgin membrane has a reduced ionic conductivity (0.27 × 10–2 S cm−1) because of the presence of ionic groups in a lower proportion connected to SPVC polymeric chains that may contain and transport ions. The water/methanol ratio and IEC values have a major impact on membrane conductivity. Increased water absorption aids in improving proton transport, while more extensive IEC encourages high proton conduction by minimizing the distance between anionic groups. PMMA integration results in the existence of a broad surface area and an ionic channel pass-way. The integration of PSS enhanced the membranes’ proton conductivity, which facilitates proton transport through the membrane due to the presence of negative charge groups (–SO3H) that can attract protons. The incorporation of PSS increased the membranes’ proton conductivity, which enhances proton transport throughout the membrane due to the presence of negatively charged groups (–SO3H) that attract protons. For SPVC/PMMA/1%PSS and SPVC/PMMA/5%PSS, respectively, the ionic conductivity rose from 1.80 × 10–2 to 4.70 × 10–2 S cm−1 as the proportion of PSS in the polyelectrolytic membrane matrix increased from 1 to 5% by weight. This pattern resembled the IEC behavior in electrolytic membranes based on SPVC, PMMA, and PSS, which increased with increasing PSS concentration [73].
The electrochemical experiments for conductivity measurements at ambient temperature employed membranes that had been submerged in 1M H2SO4 for 24 h before being washed with DI water. Figure 13 illustrates the Nyquist plots of the Pristine SPVC and SPVC/PMMA/PSS polyelectrolytic composite membranes, which are between the imaginary and real parts of the impedance (Z′′ and Z′) [74].
To determine the proton conductivity of synthetic polyelectrolytic membranes, the polarization resistance must be investigated with the Nyquist plot. The high frequency area within the plot is expanded in the figure’s inset. Traditionally, membrane ohmic resistance is represented as the high frequency intercept of the estimated Nyquist plot to the real axis (Z′). When compared to pristine SPVC, the conductivity of blended SPVC/PMMA/PSS membranes rose as the PSS concentration increased. SPVC/PMMA/4%PSS and SPVC/PMMA/5%PSS exhibited the greatest ionic conductivities of 4.00 × 10–2 and 4.70 × 10–2 S cm−1, respectively. As PSS content increases, proton conductivity also increases. The DS results did not differ significantly from other measurements, as discovered [73, 75].
Oxidative stability of membranes
The oxidation resistance significantly impacts the durability of the polymer electrolyte membrane. Figure 14 shows the outcomes of an investigation into the oxidation stability of SPVC/PMMA/PSS-based membranes in Fenton’s reagent. All developed polyelectrolytic membranes generally show significant oxidative stability by increasing the PSS conductive material’s molar ratio into a polymer matrix, reaching its maximum value of 97.58% for SPVC/PMMA/4%PSS. Compared to the pristine SPVC membrane, the modified SPVC/PMMA/PSS-based membranes showed much superior oxidative stabilities. Furthermore, this behavior is believed to be caused by oxygen-containing groups found inside the polymeric matrix, such as –SO3H (PSS) and RCOOR′ (PMMA). By forming hydrogen bonds with the polymer, these oxygen groups enhance the cross-linking density, which may impede the attack of free radicals and improve the membrane’s resistance to oxidation. Additionally, PMMA increases the surface area of the membranes, enhancing their toughness and oxidative durability [59].
Methanol permeability
Methanol permeation across PEMs during DMFC operation wastes fuel and damages the catalyst, reducing fuel cell efficiency. Proton-exchange membranes (PEMs) for DMFCs must have strong ionic conductivity and low methanol permeability.
As a result, methanol permeability was tested in a diffusion cell with the membrane clamped between two reservoirs of aqueous methanol solution and distilled water for the synthesized SPVC/PMMA/PSS blend-based membranes. The amount of penetrated methanol was plotted against time, and the findings are shown in Fig. 15. The methanol permeability coefficient may be calculated using the slope of the obtained line and the values in Table 3.
These results demonstrated that decreasing the methanol permeability was caused by increasing the PSS content in these blends. As a result, the SPVC/PMMA/PSS produced with 1 wt.% PSS had the greatest methanol permeability value of 8.53 × × 10–8 cm2/s, which was substantially lower than the comparable value for Nafion® (3.39 × 10–6 cm2/s). Despite having a higher surface roughness and pore density than pristine SPVC with a dense structure, the modified SPVC/PMMA/PSS composite-based membranes had lower methanol permeability because the presence of sulfonated polymer filler particulates caused methanol molecules to travel through a more complicated and tortuous path. This decrease in methanol permeability of the synthesized polyelectrolyte composite membranes can also be attributed to the dense and compact structure of the composite matrix, as previously demonstrated by SEM examination, as well as the high hydrophilicity of the composite membrane’s surface, which hindered methanol adsorption and thus reduced its cross-over. These findings revealed that reaching a balance between the different functional features achieved more substantial proton conduction and reduced methanol crossover simultaneously [76].
Membrane efficiency
Effective PEM for DMFC performance must have both a high proton conductivity and a low methanol permeability. To a certain extent, methanol permeability is less significant than the proton conductivity to methanol permeability ratio. The efficiency factor (φ), a percentage of ionic conductivity (σ) to methanol permeability, was used in this study to assess the rendering of the resulting membranes. Table 3 displays the acquired results. According to these results, efficiency factor values of all synthesized SPVC/PMMA/PSS membranes were more significant than that of non-functionalized pristine SPVC membrane (2.70 × 104), and this is because when the molar concentration of PSS in the polymer blend matrix rises, proton conductivity increased leading to rise efficiency factor (φ) of these blend membranes.
The highest efficiency factor value (15.81 × 105) was recorded for a mixed membrane with 4% PSS (SPVC/PMMA/4%PSS). Similarly, the efficiency factor of synthesized composite polyelectrolyte membranes created with varying loading proportions of PSS on the major polymeric components was greater than that of Nafion117 (2.60 × 105). However, the most effective polymer blend membrane was determined to be that made with SPVC/PMMA/4%PSS, and any excess in PSS over this percentage did not result in any substantial increase in membrane efficiency and so may be regarded as an unnecessary expense [35].
Conclusion
This research aimed to develop promising SPVC/PMMA/PSS composite membranes with various PSS ratios to optimize membrane characteristics and performance in DMFC operation. Sulfonated polyvinyl chloride (SPVC) was successfully synthesized, and it was then blended with polymethyl methacrylate (PMMA) and varying concentrations of polystyrene sulfonate (PSS) to create proton-conducting membranes. FT-IR, Raman spectroscopy, 1H NMR, TGA, and SEM investigations were used to survey the chemical compositional, structural characteristics, and thermal stability of the synthesized sulfonated PVC (SPVC) polymer and its blend-based polyelectrolyte membrane. FTIR substantiated the interaction of membrane components by indicating two distinct absorption bands of sulfonic groups (-SO3H) at 1093 and 1219 cm−1 as well as noticeable peaks at about 1715 cm−1 caused by vibration of carbonyl groups in the PSS part of the polymer mix. Additionally, SEM micrographs show no interphase incompatibilities or phase separation occurred in the matrix of all polymer blend proton-conducting membranes constructed utilizing the evaluated changing polymer ratios. The fabricated SPVC/PMMA/PSS composite polyelectrolyte membranes showed remarkable thermal stability, withstanding high temperatures (200–300 °C) for thermal breakdown initiation. Furthermore, the ion exchange capacity (IEC), proton conductivity, and methanol permeability of SPVC/PMMA/PSS membranes were investigated depending on PSS concentration. At room temperature, the findings showed a significant increase in proton conductivity from 1.80 × 10–2 S/cm for SPVC/PMMA/1%PSS to 4.7 × 10–2 S/cm for SPVC/PMMA/5%PSS. Methanol permeability (P = 8.53 × 10–8 cm2/s) for developed membranes was significantly lower than Nafion 117 (3.39 × 10–6 cm2/s). Additionally, the IEC of the manufactured membrane was 1.38 ± 0.7 meq g−1 for SPVC/PMMA/5%PSS compared to 0.91 meq g−1 for Nafion 117 membranes. The maximum water uptake of the synthesized membranes was 48.37 ± 2.27%, whereas Nafion 117 membrane absorption was 65.44%. In conclusion, the efficiency of fabricated sulfonated polymer composite-based membranes might be enhanced by altering the amount of PSS to tailor proton conduction and methanol barrier properties. An increase in the molar ratio of PSS did not result in a substantial rise in this efficiency factor. As a result, a molar ratio of 4 wt.% PSS is the optimal ratio for constructing innovative proton-conducting membranes and might be a low-cost contender for DMFC applications.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Yuan X-Z, Li H, Zhang S, Martin J, Wang H (2011) A review of polymer electrolyte membrane fuel cell durability test protocols. J Power Sour 196:9107–9116. https://doi.org/10.1016/j.jpowsour.2011.07.082
Tellez-Cruz MM, Escorihuela J, Solorza-Feria O, Compañ V (2021) Proton exchange membrane fuel cells (Pemfcs): advances and challenges. Polymers. https://doi.org/10.3390/polym13183064
Imaan DU, Mir FQ, Ahmad B (2021) Synthesis and characterization of a novel poly (vinyl alcohol)-based zinc oxide (PVA-ZnO) composite proton exchange membrane for DMFC. Int J Hydrog Energy 46:12230–12241. https://doi.org/10.1016/j.ijhydene.2020.05.008
Smith AT, LaChance AM, Zeng S, Liu B, Sun L (2019) Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater Sci 1:31–47. https://doi.org/10.1016/j.nanoms.2019.02.004
Kim AR, Poudel MB, Chu JY, Vinothkannan M, Santhosh Kumar R, Logeshwaran N, Park BH, Han MK, Yoo DJ (2023) Advanced performance and ultra-high, long-term durability of acid-base blended membranes over 900 hours containing sulfonated PEEK and quaternized poly(arylene ether sulfone) in H2/O2 fuel cells. Compos B Eng. https://doi.org/10.1016/j.compositesb.2023.110558
Yin C, Li J, Zhou Y, Zhang H, Fang P, He C (2018) Enhancement in Proton Conductivity and Thermal Stability in Nafion Membranes Induced by Incorporation of Sulfonated Carbon Nanotubes. ACS Appl Mater Interfaces 10:14026–14035. https://doi.org/10.1021/acsami.8b01513
An L, Zhao TS, Li YS (2015) Carbon-neutral sustainable energy technology: direct ethanol fuel cells. Renew Sustain Energy Rev 50:1462–1468. https://doi.org/10.1016/j.rser.2015.05.074
Hickner MA, Pivovar BS (2005) The chemical and structural nature of proton exchange membrane fuel cell properties. Fuel cells 5:213–229. https://doi.org/10.1002/fuce.200400064
Kim SH, Lee KH, Chu JY, Kim AR, Yoo DJ (2020) Enhanced hydroxide conductivity and dimensional stability with blended membranes containing hyperbranched paes/linear ppo as anion exchange membranes. Polymers 12:1–16. https://doi.org/10.3390/polym12123011
Rana D, Matsuura T, Narbaitz RM, Feng C (2005) Development and characterization of novel hydrophilic surface modifying macromolecule for polymeric membranes. J Memb Sci 249:103–112. https://doi.org/10.1016/j.memsci.2004.09.034
Kim AR, Vinothkannan M, Yoo DJ (2017) Sulfonated-fluorinated copolymer blending membranes containing SPEEK for use as the electrolyte in polymer electrolyte fuel cells (PEFC). Int J Hydrogen Energy 42:4349–4365. https://doi.org/10.1016/j.ijhydene.2016.11.161
Abu-Saied MA, Soliman EA, Abualnaj KM, El Desouky E (2021) Highly conductive polyelectrolyte membranes poly(Vinyl alcohol)/poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for fuel cell application. Polymers. https://doi.org/10.3390/polym13162638
Abu-Saied MA, El-Desouky EA, Soliman EA, Abd El-Naim G (2020) Novel sulphonated poly (vinyl chloride)/poly (2-acrylamido-2-methylpropane sulphonic acid) blends-based polyelectrolyte membranes for direct methanol fuel cells. Polym Test 89:106604. https://doi.org/10.1016/j.polymertesting.2020.106604
Chu W, Chu YS (2020) A highly stable Cu(OH)(2)-Poly(vinyl alcohol) nanocomposite membrane for dramatically enhanced direct borohydride fuel cell performance. J Power Sources 467:228312. https://doi.org/10.1016/j.jpowsour.2020.228312
Rana D, Kim Y, Matsuura T, Arafat HA (2011) Development of antifouling thin-film-composite membranes for seawater desalination. J Memb Sci 367:110–118. https://doi.org/10.1016/j.memsci.2010.10.050
Xu J, Sheng GP, Luo HW, Li WW, Wang LF, Yu HQ (2012) Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res 46:1817–1824. https://doi.org/10.1016/j.watres.2011.12.060
Abu-Saied MA, Khalil KA, Al-Deyab SS (2012) Preparation and characterization of poly vinyl acetate nanofiber doping copper metal. Int J Electrochem Sci 7(3):2019–2027
Rambabu G, Bhat SD (2014) Simultaneous tuning of methanol crossover and ionic conductivity of sPEEK membrane electrolyte by incorporation of PSSA functionalized MWCNTs: a comparative study in DMFCs. Chem Eng J 243:517–525. https://doi.org/10.1016/j.cej.2014.01.030
Allan JTS, Prest LE, Easton EB (2015) The sulfonation of polyvinyl chloride: synthesis and characterization for proton conducting membrane applications. J Memb Sci 489:175–182. https://doi.org/10.1016/j.memsci.2015.03.093
Yuan C, Wang Y (2020) Synthesis and characterization of a crosslinked membrane based on sulfonated poly(aryl ether sulfone) and sulfonated polyvinyl alcohol applied in direct methanol fuel cells. J Polym Res. https://doi.org/10.1007/s10965-020-02305-z
Forte MA, Silva RM, Tavares CJ, Silva E, RF, (2021) Is poly(Methyl methacrylate) (PMMA) a suitable substrate for ALD?: A review. Polymers (Basel). https://doi.org/10.3390/polym13081346
Wang A, Bao Y, Weng Z, HUANG Z, (2008) Synthesis and characterization of proton-conducting polymer electrolytes based on acrylonitrile-styrene sulfonic acid copolymer/layered double hydroxides nanocomposites. Chin J Chem Eng 16:938–943. https://doi.org/10.1016/S1004-9541(09)60020-3
Zhang Z, Liu Y, Lin S, Wang Q (2020) Preparation and properties of glutaraldehyde crosslinked poly (vinyl alcohol) membrane with gradient structure. J Polym Res 27:1–7. https://doi.org/10.1007/s10965-020-02223-0
do Nascimento FC, de Aguiar LCV, Costa LAT, Fernandes MT, Marassi RJ, Gomes A de S, de Castro JA, (2021) Formulation and characterization of crosslinked polyvinyl alcohol (PVA) membranes: effects of the crosslinking agents. Polymer Bulletin 78:917–929. https://doi.org/10.1007/s00289-020-03142-2
Yang CC, Chiu SJ, Kuo SC (2011) Preparation of poly(vinyl alcohol)/montmorillonite/poly(styrene sulfonic acid) composite membranes for hydrogen-oxygen polymer electrolyte fuel cells. Curr Appl Phys. https://doi.org/10.1016/j.cap.2010.11.043
Abu-Saied MA, Soliman EA, Al Desouki EA (2020) Development of proton exchange membranes based on chitosan blended with poly (2-acrylamido-2-methylpropane sulfonic acid) for fuel cells applications. Mater Today Commun 25:101536. https://doi.org/10.1016/j.mtcomm.2020.101536
Abu-Saied MA, Abdel-Halim ES, Fouda MM, Al-Deyab SS (2013) Preparation and characterization of iminated polyacrylonitrile for the removal of methylene blue from aqueous solutions. Int J Electrochem Sci 8(4):5121–5135
Eldin MSM, Elzatahry AA, El-Khatib KM, Hassan EA, El-Sabbah MM, Abu-Saied MA (2011) Novel grafted nafion membranes for proton-exchange membrane fuel cell applications. J Appl Polym Sci 119:120–133. https://doi.org/10.1002/app.32613
Puentes C, Joulia X, Vidal J-P, Esteban-Decloux M (2018) Simulation of spirits distillation for a better understanding of volatile aroma compounds behavior: application to armagnac production. Food Bioprod Process 112:31–62. https://doi.org/10.1016/j.fbp.2018.08.010
Kakati N, Maiti J, Das G, Lee SH, Yoon YS (2015) An approach of balancing the ionic conductivity and mechanical properties of PVA based nanocomposite membrane for DMFC by various crosslinking agents with ionic liquid. Int J Hydrogen Energy 40:7114–7123. https://doi.org/10.1016/j.ijhydene.2015.04.004
Hossain MI, Soliman MM, El-Naggar ME, Sultan MZ, Kechi A, Abdelsalam NR, Abu-Saied MA, Chowdhury M (2021) Synthesis and characterization of graphene oxide-ammonium ferric sulfate composite for the removal of dyes from tannery wastewater. J Market Res 12:1715–1727. https://doi.org/10.1016/j.jmrt.2021.03.097
Mohy Eldin MS, Tamer TM, Abu Saied MA, Soliman EA, Madi NK, Ragab I, Fadel I (2018) Click grafting of chitosan onto PVC surfaces for biomedical applications. Adv Polym Technol 37:38–49. https://doi.org/10.1002/adv.21640
Petreanu I, Ebrasu D, Sisu C, Varlam M (2012) Thermal analysis of sulfonated polymers tested as polymer electrolyte membrane for PEM fuel cells. J Therm Anal Calorim. https://doi.org/10.1007/s10973-012-2442-z
Qing X, He G, Liu Z, Yin Y, Cai W, Fan L, Fardim P (2021) Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr Polym 261:117875. https://doi.org/10.1016/j.carbpol.2021.117875
Elerian AF, Mohamed AA, Elnaggar EM, Abu-Saied MA (2024) Development of novel proton exchange membranes based on cross-linked polyvinyl alcohol (PVA)/5-sulfosalicylic acid (SSCA) for fuel cell applications. Discover Applied Sciences 6:341. https://doi.org/10.1007/s42452-024-05940-z
Eldin MSM, Abu-Saied MA, Elzatahry AA, El-Khatib KM, Hassan EA, El-Sabbah MM (2011) Novel acid-base poly vinyl chloride-doped ortho-phosphoric acid membranes for fuel cell applications. Int J Electrochem Sci 6:5417–5429. https://doi.org/10.1016/S1452-3981(23)18417-3
Abu-Saied MA, Wycisk R, Abbassy MM, Abd El-Naim G, El-Demerdash F, Youssef ME, Bassuony H, Pintauro PN (2017) Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions—Fabrication and sorption studies. Carbohydr Polym 165:149–158. https://doi.org/10.1016/j.carbpol.2016.12.039
Abu-Saied MA, Elzatahry AA, El-Khatib KM, Hassan EA, El-Sabbah MM, Drioli E, Mohy Eldin MS (2012) Preparation and characterization of novel grafted cellophane-phosphoric acid-doped membranes for proton exchange membrane fuel-cell applications. J Appl Polym Sci 123:3710–3724. https://doi.org/10.1002/app.35048
Ru C, Gu Y, Na H, Li H, Zhao C (2019) Preparation of a cross-linked sulfonated poly (arylene ether ketone) proton exchange membrane with enhanced proton conductivity and methanol resistance by introducing an ionic liquid-impregnated metal organic framework. ACS Appl Mater Interfaces 11:31899–31908. https://doi.org/10.1021/acsami.9b09183
Lee J, Salihi H, Lee J, Ju H (2022) Impedance modeling for polymer electrolyte membrane fuel cells by combining the transient two-phase fuel cell and equivalent electric circuit models. Energy. https://doi.org/10.1016/j.energy.2021.122294
Yao J, Xu G, Zhao Z, Guo J, Li S, Cai W, Zhang S (2019) An enhanced proton conductivity and reduced methanol permeability composite membrane prepared by sulfonated covalent organic nanosheets/Nafion. Int J Hydrogen Energy 44:24985–24996. https://doi.org/10.1016/j.ijhydene.2019.07.197
Hadjiivanov KI, Panayotov DA, Mihaylov MY, Ivanova EZ, Chakarova KK, Andonova SM, Drenchev NL (2021) Power of Infrared and Raman spectroscopies to characterize metal-organic frameworks and investigate their interaction with guest molecules. Chem Rev 121:1286–1424. https://doi.org/10.1021/acs.chemrev.0c00487
Farber C, Li J, Hager E, Chemelewski R, Mullet J, Rogachev AY, Kurouski D (2019) Complementarity of Raman and infrared spectroscopy for structural characterization of plant epicuticular waxes. ACS Omega 4:3700–3707. https://doi.org/10.1021/acsomega.8b03675
Awad S, Abdel-Hady EE, Mohamed HFM, Elsharkawy YS, Gomaa MM (2021) Non-fluorinated PVA/SSA proton exchange membrane studied by positron annihilation technique for fuel cell application. Polym Adv Technol 32:3322–3332. https://doi.org/10.1002/pat.5345
Gomaa MM, Hugenschmidt C, Dickmann M, Abdel-Hady EE, Mohamed HFM, Abdel-Hamed MO (2018) Crosslinked PVA/SSA proton exchange membranes: Correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys Chem Chem Phys 20:28287–28299. https://doi.org/10.1039/C8CP05301D
Kamoun EA, Youssef ME, Abu-Saied MA, Fahmy A, Khalil HF, Abdelhai F (2015) Ion Conducting Nanocomposite Membranes Based on PVA-HA-HAP for Fuel Cell Application: II. Eff Modif Agent PVA Membr Prop. https://doi.org/10.1016/S1452-3981(23)06747-0
Najafi V, Abdollahi H (2020) Internally plasticized PVC by four different green plasticizer compounds. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2020.109620
Massoumi B, Farnoudian-Habibi A, Jaymand M (2016) Chemical and electrochemical grafting of polythiophene onto poly(vinyl chloride): synthesis, characterization, and materials properties. J Solid State Electrochem 20:489–497. https://doi.org/10.1007/s10008-015-3070-x
Jia P, Zhang M, Hu L, Wang R, Sun C, Zhou Y (2017) Cardanol groups grafted on poly(vinyl chloride)-synthesis, performance and plasticization mechanism. Polymers (Basel). https://doi.org/10.3390/polym9110621
Farrokhzad H, Kikhavani T, Monnaie F, Ashrafizadeh SN, Koeckelberghs G, Van Gerven T, Van der Bruggen B (2015) Novel composite cation exchange films based on sulfonated PVDF for electromembrane separations. J Memb Sci 474:167–174. https://doi.org/10.1016/j.memsci.2014.10.002
Ahmadi B, Seyed Dorrji MS, Kianfar M, Rasoulifard MH, Ahmadi A (2022) A novel multilayer thin-film membrane with high durability: preparation, characterization, performance investigation. New J Chem 46:15006–15015. https://doi.org/10.1039/D2NJ01170K
Popova M, Lazarova H, Szegedi A, Mihályi MR, Rangus M, Likozar B, Dasireddy VDBC (2018) Renewable glycerol esterification over sulfonic-modified mesoporous silicas. J Serb Chem Soc 83:39–50. https://doi.org/10.2298/JSC170306071P
Sepehrianazar A, Güven O (2022) Synthesis and characterization of (allylamine hydrochloride-vinyl sulfonic acid) copolymer and determination of monomer reactivity ratios. J Polym Res 29:330. https://doi.org/10.1007/s10965-022-03106-2
Socrates G (2004) Infrared and Raman characteristic group frequencies: tables and charts. John Wiley & Sons, Hoboken
She J, Gao H, Song Z, Shi L, Li J, Lu X, Wu C (2023) Improvement of persistent hydrophilicity and pore uniformity of polyvinyl chloride ultrafiltration membranes by in-situ crosslinking reaction assisted phase separation. J Memb Sci. https://doi.org/10.1016/j.memsci.2023.121884
Deshmukh K, Joshi GM (2014) Thermo-mechanical properties of poly (vinyl chloride)/graphene oxide as high performance nanocomposites. Polym Test 34:211–219. https://doi.org/10.1016/j.polymertesting.2014.01.015
Liu B, Chen C, Zhang W, Crittenden J, Chen Y (2012) Low-cost antifouling PVC ultrafiltration membrane fabrication with Pluronic F 127: Effect of additives on properties and performance. Desalination 307:26–33. https://doi.org/10.1016/j.desal.2012.07.036
Elerian AF, Abu-Saied MA, Abd-Elnaim GH, Elnaggar EM (2023) Development of polymer electrolyte membrane based on poly (Vinyl Chloride)/graphene oxide modified with zirconium phosphate for fuel cell applications. J Polym Res 30:6. https://doi.org/10.1007/s10965-022-03317-7
Mahmoud A, Fahmy A, Naser A, Saied MA (2022) Novel sulfonated poly (vinyl alcohol)/carboxy methyl cellulose/acrylamide-based hybrid polyelectrolyte membranes. Sci Rep. https://doi.org/10.1038/s41598-022-26489-0
Sahu AK, Selvarani G, Bhat SD, Pitchumani S, Sridhar P, Shukla AK, Narayanan N, Banerjee A, Chandrakumar N (2008) Effect of varying poly(styrene sulfonic acid) content in poly(vinyl alcohol)-poly(styrene sulfonic acid) blend membrane and its ramification in hydrogen-oxygen polymer electrolyte fuel cells. J Memb Sci 319:298–305. https://doi.org/10.1016/j.memsci.2008.04.004
Knauth P, Hou H, Bloch E, Sgreccia E, Di Vona ML (2011) Thermogravimetric analysis of SPEEK membranes: Thermal stability, degree of sulfonation and cross-linking reaction. J Anal Appl Pyrolysis 92:361–365. https://doi.org/10.1016/j.jaap.2011.07.012
Pirali-Hamedani M, Mehdipour-Ataei S (2017) Effect of sulfonation degree on molecular weight, thermal stability, and proton conductivity of poly(arylene ether sulfone)s membrane. Des Monomers Polym 20:54–65. https://doi.org/10.1080/15685551.2016.1231035
Dutta K, Das S, Kundu PP (2015) Partially sulfonated polyaniline induced high ion-exchange capacity and selectivity of Nafion membrane for application in direct methanol fuel cells. J Memb Sci 473:94–101. https://doi.org/10.1016/j.memsci.2014.09.010
Khan S, Ranjha NM (2014) Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym Bull 71:2133–2158. https://doi.org/10.1007/s00289-014-1178-2
Rana D, Cho K, Woo T, Lee BH, Choe S (1999) Blends of ethylene 1-octene copolymer synthesized by Ziegler-Natta and metallocene catalysts. I. Thermal and mechanical properties. J Appl Polym Sci 74:1169–1177. https://doi.org/10.1002/(SICI)1097-4628(19991031)74:5%3c1169::AID-APP13%3e3.0.CO;2-W
Zakaria Z, Kamarudin SK (2020) A review of quaternized polyvinyl alcohol as an alternative polymeric membrane in DMFCs and DEFCs. Int J Energy Res 44:6223–6239
Bhattacharya M (2016) Polymer nanocomposites—a comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 9:262. https://doi.org/10.1002/er.5314
Abu-Saied MA, Taha TH, El-Deeb NM, Hafez EE (2018) Polyvinyl alcohol/Sodium alginate integrated silver nanoparticles as probable solution for decontamination of microbes contaminated water. Int J Biol Macromol 107:1773–1781. https://doi.org/10.1016/j.ijbiomac.2017.10.047
Sun F, Zhou H, Lee J (2011) Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater 7:3813–3828. https://doi.org/10.1016/j.actbio.2011.07.002
Simari C, Enotiadis A, Nicotera I (2020) Transport properties and mechanical features of sulfonated polyether ether ketone/organosilica layered materials nanocomposite membranes for fuel cell applications. Membranes. https://doi.org/10.3390/membranes10050087
Krainer S, Hirn U (2021) Contact angle measurement on porous substrates: Effect of liquid absorption and drop size. Colloids Surf, A 619:126503. https://doi.org/10.1016/j.colsurfa.2021.126503
Neelakandan S, Rana D, Matsuura T, Muthumeenal A, Kanagaraj P, Nagendran A (2014) Fabrication and electrochemical properties of surface modified sulfonated poly (vinylidenefluoride-co-hexafluoropropylene) membranes for DMFC application. Solid State Ion 268:35–41. https://doi.org/10.1016/j.ssi.2014.09.027
Yang C-C, Lue SJ, Shih J-Y (2011) A novel organic/inorganic polymer membrane based on poly (vinyl alcohol)/poly (2-acrylamido-2-methyl-1-propanesulfonic acid/3-glycidyloxypropyl trimethoxysilane polymer electrolyte membrane for direct methanol fuel cells. J sPower Sour 196:4458–4467. https://doi.org/10.1016/j.jpowsour.2011.01.051
Fan X, Li Z, Wang S, Liu L, Liu P, Chen F, Zheng X (2019) Electrochemical impedance biosensor for the determination of lipopolysaccharide using peptide as the recognition molecule. J Braz Chem Soc 30:1762–1768. https://doi.org/10.21577/0103-5053.20190081
Najafi B, Bonomi P, Casalegno A, Rinaldi F, Baricci A (2020) Rapid fault diagnosis of PEM fuel cells through optimal electrochemical impedance spectroscopy tests. Energies. https://doi.org/10.3390/en13143643
Rezaei Niya SM, Hoorfar M (2015) Process modeling of electrodes in proton exchange membrane fuel cells. J Electroanal Chem 747:112–122. https://doi.org/10.1016/j.jelechem.2015.04.015
Altaf F, Gill R, Batool R, Drexler M, Alamgir F, Abbas G, Jacob K (2019) Proton conductivity and methanol permeability study of polymer electrolyte membranes with range of functionalized clay content for fuel cell application. Eur Polym J 110:155–167. https://doi.org/10.1016/j.eurpolymj.2018.11.027
Acknowledgements
The authors extend their appreciation to the Science and Technology Development Fund (STDF) for funding this work through the General Research Project under grant number (46947).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Science and Technology Development Fund, 46947.
Author information
Authors and Affiliations
Contributions
Ahmed F. Elerian contributed to methodology, investigation, formal analysis, and writing; Abdallah A. Mohamed contributed to conceptualization, writing, visualization, and formal analysis; Elsayed M. Elnaggar contributed to data curation, validation, review and editing; G. Abd El-Naeem contributed to writing, visualization, and formal analysis, and M. A. Abu-Saied contributed to conceptualization, formal analysis, data curation, Supervision, review, and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Consent to participant
All authors participate in this work.
Consent for publication
All authors agree to publish this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elerian, A.F., Mohamed, A.A., Elnaggar, E.M. et al. Investigation on sulfonated PVC/polymethyl methacrylate (PMMA)/polystyrene sulfonate (PSS) polymer blends as proton-conducting membrane. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05487-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05487-4