Abstract
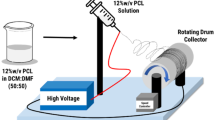
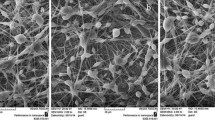
The demand for wound care is increasing globally and the traditional wound dressings offer limited support to the healing process, failing to meet the requirements of current healthcare system. Drug-controlled release nanotechnology has garnered interest recently in the field of biomedicine. Electrospun nanofibers are considered as an advanced wound dressing due to their unique structure and a biological function similar to the extracellular matrix. In this study, a polycaprolactone (PCL)-polyethylene glycol (PEG) loaded with nisin was developed by electrospinning technique. The fabricated nanofiber was characterized by scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). The degradation index, in vitro release studies, antioxidant activity, and cell cytotoxicity analyses of the nanofibers were also performed. The antibacterial activity of the nanofiber was evaluated against wound causing skin pathogens like Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, and Klebsiella pneumoniae. The FTIR data demonstrate the interactions between PCL, PEG, and nisin. The presence of nisin in the fiber was confirmed by the characteristic bands of nisin at 3398.63, 1643.23, and 1242.17 cm−1. The nanofibers exhibited maximum controlled nisin release of 94.3% at 24 h. The nisin-loaded nanofibers exhibited antibacterial activity against all the indicator organisms used in the study. About 78% viability was observed on nisin-loaded nanofibers during the MTT assay. The results indicated that the fabricated antibacterial nanofiber has the potential for wound healing application and the treatment of bacterial infections in wounds.









Similar content being viewed by others
Data and code availability
Not applicable.
References
Momoh FU, Boateng JS, Richardson SCW et al (2015) Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int J Biol Macromol 81:137–150
Saghazadeh S, Rinoldi C, Schot M et al (2018) Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev 127:138–166
Augustine R, Kalarikkal N, Thomas S (2016) Electrospun PCL membranes incorporated with biosynthesized silver nanoparticles as antibacterial wound dressings. Appl Nanosci 6:337–344
Kulkarni D, Musale S, Panzade P et al (2022) Surface functionalization of nanofibers: the multifaceted approach for advanced biomedical applications. Nanomaterials 12:3899
Felgueiras HP, Amorim MTP (2017) Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf B Biointerfaces 156:133–148
Garkal A, Kulkarni D, Musale S et al (2021) Electrospinning nanofiber technology: a multifaceted paradigm in biomedical applications. New J Chem 45:21508–21533
Zhao R, Li X, Sun B et al (2014) Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int J Biol Macromol 68:92–97
Çalamak S, Erdoğdu C, Özalp M, Ulubayram K (2014) Silk fibroin based antibacterial bionanotextiles as wound dressing materials. Mater Sci Eng, C 43:11–20
Hu X, Liu S, Zhou G et al (2014) Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release 185:12–21. https://doi.org/10.1016/j.jconrel.2014.04.018
Rošic R, Kocbek P, Baumgartner S, Kristl J (2011) Electro-spun hydroxyethyl cellulose nanofibers: the relationship between structure and process. J Drug Deliv Sci Technol 21:229–236
Pant HR, Neupane MP, Pant B et al (2011) Fabrication of highly porous poly (ɛ-caprolactone) fibers for novel tissue scaffold via water-bath electrospinning. Colloids Surf B Biointerfaces 88:587–592
Beachley V, Wen X (2010) Polymer nanofibrous structures: fabrication, biofunctionalization, and cell interactions. Prog Polym Sci 35:868–892
Khil M, Cha D, Kim H et al (2003) Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res Part B Appl Biomater 67:675–679
Dash TK, Konkimalla VB (2012) Poly-є-caprolactone based formulations for drug delivery and tissue engineering: a review. J Control Release 158:15–33
Luong-Van E, Grøndahl L, Chua KN et al (2006) Controlled release of heparin from poly (ε-caprolactone) electrospun fibers. Biomaterials 27:2042–2050
Hrib J, Sirc J, Hobzova R et al (2015) Nanofibers for drug delivery–incorporation and release of model molecules, influence of molecular weight and polymer structure. Beilstein J Nanotechnol 6:1939–1945
Pawar R, Pathan A, Nagaraj S et al (2023) Polycaprolactone and its derivatives for drug delivery. Polym Adv Technol 34:3296–3316
Bui HT, Chung OH, Dela Cruz J, Park JS (2014) Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol Res 22:1288–1296
Riaz T, Khenoussi N, Rata DM et al (2021) Blend electrospinning of poly (ɛ-caprolactone) and poly (ethylene glycol-400) nanofibers loaded with ibuprofen as a potential drug delivery system for wound dressings. Autex Res J 23:66–76
Dehghan F, Gholipour-Kanani A, Kamali Dolatabadi M, Bahrami SH (2022) Nanofibrous composite from polycaprolactone-polyethylene glycol-aloe vera as a promising scaffold for bone repairing. J Appl Polym Sci 139:e52463
Malvisi M, Stuknytė M, Magro G et al (2016) Antibacterial activity and immunomodulatory effects on a bovine mammary epithelial cell line exerted by nisin A-producing Lactococcus lactis strains. J Dairy Sci 99:2288–2296
Zhao P, Xue Y, Gao W et al (2018) Bacillaceae-derived peptide antibiotics since 2000. Peptides 101:10–16
Andersson DI, Hughes D, Kubicek-Sutherland JZ (2016) Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist Updat 26:43–57
Heunis TDJ, Smith C, Dicks LMT (2013) Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob Agents Chemother 57:3928–3935
Gunes OC, Ziylan Albayrak A (2021) Antibacterial polypeptide nisin containing cotton modified hydrogel composite wound dressings. Polym Bull 78:6409–6428
Dart A, Sarviya N, Babaie A et al (2023) Highly active nisin coated polycaprolactone electrospun fibers against both Staphylococcus aureus and Pseudomonas aeruginosa. Biomater Adv 154:213641
Arthanari S, Mani G, Jang JH et al (2016) Preparation and characterization of gatifloxacin-loaded alginate/poly (vinyl alcohol) electrospun nanofibers. Artif Cells Nanomed Biotechnol 44:847–852
Wang H, She Y, Chu C et al (2015) Preparation, antimicrobial and release behaviors of nisin-poly (vinyl alcohol)/wheat gluten/ZrO 2 nanofibrous membranes. J Mater Sci 50:5068–5078
Ibrahim S, Rezk MY, Ismail M et al (2020) Coaxial nanofibers outperform uniaxial nanofibers for the loading and release of pyrroloquinoline quinone (PQQ) for biomedical applications. Nanoscale Adv 2:3341–3349
Vatankhah E (2018) Rosmarinic acid-loaded electrospun nanofibers: in vitro release kinetic study and bioactivity assessment. Eng Life Sci 18:732–742
Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T et al (2013) Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm 452:333–343
Golkar P, Kalani S, Allafchian AR et al (2019) Fabrication and characterization of electrospun Plantago major seed mucilage/PVA nanofibers. J Appl Polym Sci 136:47852
Chizari M, Khosravimelal S, Tebyaniyan H et al (2022) Fabrication of an antimicrobial peptide-loaded silk fibroin/gelatin bilayer sponge to apply as a wound dressing; an in vitro study. Int J Pept Res Ther 28:1–13
Ali S, Khatri Z, Oh KW et al (2014) Preparation and characterization of hybrid polycaprolactone/cellulose ultrafine fibers via electrospinning. Macromol Res 22:562–568
Benkaddour A, Jradi K, Robert S, Daneault C (2013) Grafting of polycaprolactone on oxidized nanocelluloses by click chemistry. Nanomaterials 3:141–157
Vrandečić NS, Erceg M, Jakić M, Klarić I (2010) Kinetic analysis of thermal degradation of poly (ethylene glycol) and poly (ethylene oxide) s of different molecular weight. Thermochim Acta 498:71–80
Zeng J, Yang L, Liang Q et al (2005) Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J Control Release 105:43–51
Taghipour-Sabzevar V, Sharifi T, Bagheri-Khoulenjani S et al (2020) Targeted delivery of a short antimicrobial peptide against CD44-overexpressing tumor cells using hyaluronic acid-coated chitosan nanoparticles: an in vitro study. J Nanopart Res 22:1–16
Kyzioł A, Michna J, Moreno I et al (2017) Preparation and characterization of electrospun alginate nanofibers loaded with ciprofloxacin hydrochloride. Eur Polym J 96:350–360
Fathi HA, Abdelkader A, AbdelKarim MS et al (2020) Electrospun vancomycin-loaded nanofibers for management of methicillin-resistant Staphylococcus aureus-induced skin infections. Int J Pharm 586:119620
Wang Z, Wang H, Xiong J et al (2021) Fabrication and in vitro evaluation of PCL/gelatin hierarchical scaffolds based on melt electrospinning writing and solution electrospinning for bone regeneration. Mater Sci Eng C 128:112287
Kohsari I, Shariatinia Z, Pourmortazavi SM (2016) Antibacterial electrospun chitosan–polyethylene oxide nanocomposite mats containing bioactive silver nanoparticles. Carbohydr Polym 140:287–298
Alizadeh S, Farshi P, Farahmandian N et al (2023) Synergetic dual antibiotics-loaded chitosan/poly (vinyl alcohol) nanofibers with sustained antibacterial delivery for treatment of XDR bacteria-infected wounds. Int J Biol Macromol 229:22–34
Dheraprasart C, Rengpipat S, Supaphol P, Tattiyakul J (2009) Morphology, release characteristics, and antimicrobial effect of nisin-loaded electrospun gelatin fiber mat. J Food Prot 72:2293–2300
Korsmeyer RW, Gurny R, Doelker E et al (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
Jeckson TA, Neo YP, Sisinthy SP et al (2021) Formulation and characterisation of deferoxamine nanofiber as potential wound dressing for the treatment of diabetic foot ulcer. J Drug Deliv Sci Technol 66:102751
Aragon J, Costa C, Coelhoso I et al (2019) Electrospun asymmetric membranes for wound dressing applications. Mater Sci Eng C 103:109822
Zhao Y, Wang H, Zou X et al (2022) Antibacterial vancomycin@ ZIF-8 loaded PVA nanofiber membrane for infected bone repair. Int J Mol Sci 23:5629
Yang Q, Xie Z, Hu J, Liu Y (2021) Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Mater Sci Eng, C 128:112319
Garcia EJ, Oldoni TLC, de Alencar SM et al (2012) Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J 23:22–27
Molyneux P (2004) The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 26:211–219
Solaberrieta I, Jiménez A, Cacciotti I, Garrigós MC (2020) Encapsulation of bioactive compounds from aloe vera agrowastes in electrospun poly (ethylene oxide) nanofibers. Polymers 12:1323
Nejatzadeh-Barandozi F (2013) Genetic diversity in Aloe vera accessions from Iran based on agro-morphological, phytochemical and random amplified polymorphic DNA (RAPD) markers. J Med Plant Res 7:1869–1877
Shen Q, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol 62:1097–1103
Aydogdu A, Sumnu G, Sahin S (2019) Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr Polym 208:241–250. https://doi.org/10.1016/j.carbpol.2018.12.065
Tajfiroozeh F, Moradi A, Shahidi F et al (2023) Fabrication and characterization of gallic-acid/nisin loaded electrospun core/shell chitosan/polyethylene oxide nanofiberous membranes with free radical scavenging capacity and antimicrobial activity for food packing applications. Food Biosci 53:102529
Ahmed S, Keniry M, Padilla V et al (2023) Development of pullulan/chitosan/salvianolic acid ternary fibrous membranes and their potential for chemotherapeutic applications. Int J Biol Macromol 250:126187
Dos Santos CA, Dos Santos GR, Soeiro VS et al (2018) Bacterial nanocellulose membranes combined with nisin: a strategy to prevent microbial growth. Cellulose 25:6681–6689
Schnell E, Klinkhammer K, Balzer S et al (2007) Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 28:3012–3025. https://doi.org/10.1016/j.biomaterials.2007.03.009
Albaugh VL, Mukherjee K, Barbul A (2017) Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J Nutr 147:2011–2017. https://doi.org/10.3945/jn.117.256404
Asadi N, Mehdipour A, Ghorbani M et al (2021) A novel multifunctional bilayer scaffold based on chitosan nanofiber/alginate-gelatin methacrylate hydrogel for full-thickness wound healing. Int J Biol Macromol 193:734–747. https://doi.org/10.1016/j.ijbiomac.2021.10.180
Ran L, Peng S-Y, Wang W et al (2022) In vitro and in vivo evaluation of the bioactive nanofibers-encapsulated benzalkonium bromide for accelerating wound repair with MRSA skin infection. Int J Nanomedicine 17:4419–4432
Shi C, Wang C, Liu H et al (2020) Selection of appropriate wound dressing for various wounds. Front Bioeng Biotechnol 8:182
Anumudu C, Hart A, Miri T, Onyeaka H (2021) Recent advances in the application of the antimicrobial peptide nisin in the inactivation of spore-forming bacteria in foods. Molecules 26. https://doi.org/10.3390/molecules26185552
Gut IM, Blanke SR, van der Donk WA (2011) Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem Biol 6:744–752. https://doi.org/10.1021/cb1004178
Koprivnjak T, Peschel A (2011) Bacterial resistance mechanisms against host defense peptides. Cell Mol Life Sci 68:2243–2254
Yu L, Dou S, Ma J et al (2021) An antimicrobial peptide-loaded chitosan/polyethylene oxide nanofibrous membrane fabricated by electrospinning technology. Front Mater 8. https://doi.org/10.3389/fmats.2021.650223
Wenzel M, Senges CHR, Zhang J et al (2015) Antimicrobial peptides from the aurein family form ion-selective pores in Bacillus subtilis. ChemBioChem 16:1101–1108
Acknowledgements
The authors of this study express their gratitude to SRM Central Instrumentation Facility (SCIF), Nanotechnology Research Center (NRC), Department of Translational Medicine and Research (TMR), Research Facility (I, II, III), Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology (SRMIST), Kattankalathur, Tamil Nadu for providing the instrument facilities for various analysis. We are deeply grateful to Synkromax Biotech Pvt Ltd, Chennai, Tamil Nadu for providing valuable suggestions and the apparatus for electrospinning.
Funding
The authors confirm that they have not received any funds, grants, or other assistance for this research work.
Author information
Authors and Affiliations
Contributions
SS contributed to conceptualization, methodology, validation, formal analysis, investigation, resources, software, data curation, writing-original draft preparation. SR contributed to review and editing, visualization, and supervision of project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silpa, S., Rupachandra, S. Fabrication and in vitro characterization of nisin-incorporated PCL/PEG electrospun nanofibers for wound dressing applications. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05305-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05305-x