Abstract
Naringenin imprinted cryogel membranes (Nar-ICMs) were constructed for the selective separation of naringenin from the natural media. Acrylamide was used as functional monomer, while 2-hydroxyethyl methacrylate was used as co-monomer. Nar-ICMs were subjected to some characterization analyses, e.g., contact angle measurement, swelling tests, FTIR and SEM. Selectivity studies of Nar-ICMs were carried out both in aqueous media and natural orange juice. In selectivity studies, gallic and caffeic acid molecules were used as competitor agents due to their structural similarity to naringenin. Some results obtained as follows: Contact angle values for Nar-ICMs and non-imprinted cryogel membranes (non-ICMs) were found to be 58.5° and 71.8°, respectively. The maximum adsorption capacities of Nar-ICMs and non-ICMs for naringenin were found to be 66.5 and 14 mg/g, respectively at an initial concentration of 2 mg/mL. The qmax and Kd values of the high-affinity binding sites of the obtained Scatchard plot were found to be 0.126 mmol/g (34.4 mg/g) and 0.16 mM (1.6.10–4 M), respectively. The selectivity of Nar-ICMs for naringenin was found as 1.99 and 2.26 times high when compared to gallic and caffeic acids, respectively. The adsorption of naringenin from natural orange juice with Nar-ICMs was found to be 843 µg/g.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many positive effects of consumption of naringin and naringenin, which are the main bioactive polyphenols in citrus fruits and are very similar to each other with active structure, on human health have been known since ancient times [1]. A lot of scientific publications have been given to the literature that these flavonoids have many biochemical activities such as a powerful antioxidant, anticancer, antiviral, antitumor and anti-inflammatory effects besides their cardio and neurovascular diseases treatment feature [2,3,4,5]. After naringin is taken into the body with the diet, it is broken down into naringenin and sugar parts by the intestinal microflora and then, absorbed. Although naringenin is only an aglycone form of naringin, it is more dominant with regard to biochemical activities because of steric hindrance of sugar part of naringin [6]. Naringenin can be obtained as a low amount of natural molecule, as well as by hydrolysis of naringin enzymatically or hydrolysis with different chemicals in the production of naringenin for pharmaceutical purposes. However, excessive use of chemicals in these methods can be an environmental threat as well as creating purity problems. Therefore, it is important to use environmentally friendly and economical methods for the production of naringenin [3]. Here, regarding the solution of the problems mentioned above, naringenin imprinted cryogel membranes (Nar-ICMs) were prepared for the selective separation of naringenin from the natural media under the eco-friendly circumstances.
Molecular imprinted polymers (MIPs) are also defined as synthetic models of antibody-antigen systems, which are biological molecules. MIPs operate on a “lock and key” model in terms of recognize selectively the template molecule that is polymerized in the synthesis process [7]. Despite their potential antigen–antibody system-based work, MIPs also have advantages such as low cost and resistance to harsh environmental conditions [8]. For example, while the operating temperatures of biological structures are limited to the physiological temperatures of living things, MIPs can operate in a wider range. In addition, while the storage conditions and shelf life of biological receptors are limited after isolation, MIPs are not much affected by these kinds of environmental conditions [9]. MIPs, which are obtained by using suitable functional monomer, cross-linker and template molecule, become ready for use by removing the template molecule from the synthesized polymer under appropriate conditions after polymerization [10]. The studies of MIPs, which are carried out at the academic level in different fields such as purification and detection [11,12,13,14], also show themselves in the industrial dimension [15].
In separation science, many kinds of adsorbents in different shapes and sizes have been prepared and used for analytical purposes and purification of biomolecules. However, among these, microporous cryogels have come to the forefront with their superior flow dynamics without mass transfer restriction, especially in viscous environments, and have found wide use in fields such as chemistry, biology and biotechnology [16,17,18,19].
Herein, naringenin imprinted cryogel membranes were synthesized and tested with regard to their separation capacity and selectivity for naringenin from aqueous solution and natural media. For the polymerization, acrylamide, 2-hydroxyethyl methacrylate and N,N′-and methylenebis(acrylamide) were used as functional monomer, co-monomer and cross-linker, respectively. For the selectivity studies, gallic and caffeic acid molecules were used as competitor agents due to their structural similarity to naringenin. To the best of our knowledge, cryogel-based naringenin imprinted polymeric membranes are the first study for the selective separation of naringenin from a natural environment.
Experimental
Materials
Naringenin (Nar), callic acid, caffeic Acid, Acrylamide (AAm), 2-hydroxyethyl methacrylate (HEMA), N,N′-Methylenebis(acrylamide) (MAAm), N,N,N′,N′-Tetramethyl ethylenediamine (TEMED), ammonium persulfate (APS), ethanol and methanol were purchased from Sigma (St. Louis, USA). Other chemicals used were of analytical grade and were obtained from Merck AG (Darmstadt, Germany).
Determination of optimal ratios for naringenin and functional monomer
Before preparation of a molecular imprinted polymer, determination of functional monomer ratio around template molecule has a crucial important. In this study, in order to determine the appropriate interaction ratio for functional monomer and template molecule (Nar) to synthesize Nar-imprinted cryogel membranes (Nar-ICMs), it is primarily necessary to find a common solvent medium in which all active substances will dissolve. In this context, after determining ethanol is the common solvent for naringenin and AAm, pre-organization complexes were prepared by dissolving Nar/AAm (1/2, 1/3, 1/4; mol/mol) in ethanol at different rates. And the graphics obtained by scanning the prepared pre-complexes at a wavelength of 280–350 nm using a UV–Vis spectrophotometer were evaluated, and given in Fig. 1.
In the results of the spectrophotometric analyses of the aforementioned precomplexing ratios given in Fig. 1, the shift of the maximum wavelength to the right means that the molecule becomes more stable in energy. In addition, the decrease in light intensity shows that the electrons on the template molecule are stable. In this case, a 1/3 ratio (0.1 mmol Nar/0.3 mmol AAm) was predicted for the optimal template molecule-functional monomer interaction ratio, and studies were continued with this ratio which is schematized as an inset in Fig. 1
Preparation of naringenin imprinted cryogel membranes
Experimental procedures used by some researchers were concinnated to synthesize Nar-ICMs [20,21,22]. Briefly, 0.5 mL of pre-complexation mixture including 0.1 mmol of Nar and 0.3 mmol of AAm, which are incubated in ethanol for 30 min, was poured into a solution composed of 1.8 mmol MBAAm and 1.5 mmol of HEMA in 4 mL of water. After degassing process by using N2 to remove O2 from monomer mixture, all mixture was transferred into a plastic syringe. After adding 15 μL (10% (w/v) APS to produce free radical, and 20 μL TEMED as catalyst into the syringe, all mixture was vortexed and put into a refrigerator at − 12 °C for 24 h. After thawing the Nar-ICMs, which was removed from the freezer at the end of 24 h, it was washed several times in the order of distilled water-ethanol-pure water to remove unreacted monomer and other polymerization residues. The washing efficiency of Nar-ICMs was followed spectrophotometrically. Nar-ICMs, free from impurities, was incubated in a solution containing 0.02% sodium azide at 4 °C until experimental uses. It should also be noted that non-imprinted cryogel membranes (non-ICMs) were also synthesized without naringenin and subjected to all procedure made for Nar-ICMs.
Characterization studies
The synthesized Nar-ICMs were characterized with regard to some parameters such as contact angle measurement (Krüss DSA100 (Hamburg, Germany) to get some information about surface hydrophilicity, swelling tests, scanning electron microscopy for information on surface morphology (SEM, EVO LS 10 ZEISS 5600 SEM, Tokyo, Japan), and Fourier transform infrared spectroscopy (FTIR) to get information about the chemical structure of membranes (Perkin Elmer, Spectrum 100, USA) analyses. Swelling tests were realized by considering two equations given below.
Nar-ICMs and non-ICMs were subjected to two different tests in terms of water retention and porosity. In the first of these, the free water amount (FWA) is calculated, and the porosity ratio is found, while in the second one, the total water amount (TWA) is calculated. For this purpose, firstly, the cryogel membrane samples saturated with water were put in a graduated vessel with a known initial volume (Vi), and the change in volume was measured (Vf). Volume of the cryogel was calculated from the difference (i.e., Vo = Vf–Vi). Then, calculations were made for FWA and TWA. For FWA, after the weight of the water-saturated cryogel membranes were taken (mw), They were squeezed between two fingers, and water was removed. In the second case, the squeezed membrane weights were taken (ms), and they were subjected to two types of tests. After the water was removed at 60 °C (md), the obtained values were evaluated by Eq. 1 and Eq. 2. Here, ρ is the density of water.
Naringenin adsorption studies
Adsorption studies of naringenin on Nar-ICMs were carried out in batch-wise, and in two different environments, e.g., aqueous and natural environments. In experimental studies performed in aqueous media, the effects of some parameters such as concentration, pH, temperature and ionic strength in the adsorption medium on naringenin adsorption to Nar-ICMs were investigated.
For investigation of the concentration effect on Nar-ICMs, naringenin solutions at different concentrations in the range of 0.1–3 mg/mL were interacted with Nar-ICMs in a temperature-controlled shaking water bath. In order to examine the effect of ambient pH on naringenin adsorption, naringenin solutions with an initial concentration of 0.1 mg/mL were prepared with 0.05 M acetate buffer at different pHs (e.g., pH 3, 4, 5 and 6). Nar-ICMs were added to the prepared solutions at different pHs, and the adsorption process was initiated in shaking water bath. In order to view the effect of ambient temperature on naringenin adsorption, solutions with initial naringenin concentration of 0.1 mg/mL (0.05 M acetate buffer at pH 5) were interacted with Nar-ICMs at three different ambient temperatures such as 10, 20 and 30 °C. In order to study the effect of medium ionic strength on naringenin adsorption, solutions having 0.1 mg/mL naringenin (0.05 M acetate buffer at pH 5) and four different NaCl concentrations in the range of 0.1–0.4 mg/mL were prepared and interacted with Nar-ICMs at three different temperatures such as 10, 20 and 30 °C. All studies mentioned above were performed for non-ICMs too. All sample readings were performed at 290 nm in a UV–Vis spectrophotometer. The amount of naringenin bound to the cryogel membranes was calculated using Eq. 3.
Here, the amount of naringenin adsorbed per gram (q, mg/g); Ci and Cf represent the concentration of naringenin in the initial and final solutions (mg/mL), respectively, V is the solution volume (mL), and m is the cryogel sample weight used (g).
Selectivity studies
In order to show the selectivity of naringenin for Nar-ICMs, gallic and caffeic acid molecules having structural similarity to naringenin were used as competitor agents in selectivity studies. In those studies, two sets of gallic and caffeic acid solutions were prepared at a concentration of 0.1 mg/mL, and the interactions were initiated in a shaking water bath by adding Nar-ICMs and non-ICMs to the prepared solutions. Spectrophotometric values were read at 212 nm wavelengths for gallic acid and 315 nm for caffeic acid, respectively. Each study was repeated in 3 replicates and the mean value was calculated.
The selectivity coefficient (k) and relative selectivity coefficient (k′) were also calculated to determine the specificity of Nar-ICMs and non-ICMs. For these purposes, distribution coefficients (KD) for naringenin, gallic acid and caffeic acid molecules were calculated by using Eq. 4. In Eq. 4, KD represents the distribution coefficient (mL/g) while Ci and Cf represent the concentrations of molecules in the initial and final interaction medium (mg/mL), respectively. V is the sample volume (mL) and m is the weight of the adsorbent (g).
The selectivity coefficients (k) for naringenin in the presence of competitor molecules were obtained by using Eq. 5.
The k values obtained for Nar-ICMs and non-ICMs allowing interpretation of the imprinting selectivity are known as relative selectivity coefficient (k′) and is calculated by using Eq. 6.
Adsorption of naringenin from natural source
The obtained fresh orange juice (OJ) was separated from the pulp by centrifugation, and three different studies were performed with undiluted, 1/2 and 1/10 diluted OJs. Dilution was done with deionized water. After adding Nar-ICMs and non-ICMs separately to 20 mL of each of these solutions, adsorption process was started in a shaking water bath. The obtained values were read at 290 nm and evaluated according to Eq. 3.
Removal of naringenin from Nar-ICMs
In order to effectively desorb naringenin from Nar-ICMs, some solutions, e.g., pure water, 40 mg/mL NaCl solution, pure ethanol, pure methanol, mixture of 10% acetic acid and 90% ethanol, mixtures of 20% acetic acid and 80% ethanol were used as desorption agents. Certain amounts of Nar-ICM samples were weighed and added to 20 mL desorption solutions and then, interacted for 30 min in a shaking water bath at 20 º C room temperature. Values were read spectrophotometrically at 290 nm wavelength and were evaluated according to Eq. 3.
Results and discussions
Characterization studies
Free water amount (FWA) and total water amount (TWA) of imprinted and non-imprinted membranes computed to determine the porosity of structures. While the FWA of Nar-ICMs and non-ICMs were found as 50% and 54%, respectively, those results were calculated for TWA as 84% and 92% for Nar-ICMs and non-ICMs, respectively. By evaluating the results, the total water capacities of the small pores in Nar-ICMs and non-ICMs were calculated to be 34% and 38%, respectively. The presence of naringenin molecule, which has hydrophobic character in the imprinted structure, decreased the water holding ability of the polymer by 4%. In general, the approximately 50% macroporous structure of the imprinted membrane allows easy mass transfer in the cryogel membrane without blocking the membrane structures, especially even working with raw fruit juices.
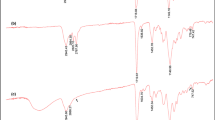
FTIR studies based on the determination of functional groups of Nar-ICMs, non-ICMs and naringenin structures are given in Fig. 2.
In Fig. 2a–c, bands observed around 3280 cm−1 were because of –OH groups in structures. In Fig. 2a and c, peaks observed between 1498 and 1519 cm−1 was attributed to the C=C aromatic stretching band in naringenin. The C–H stretching bands of aromatic compounds in naringenin were observed around 3100 cm−1 in Fig. 2a and c. The carbonyl stretching band (amide I) observed at 1650 in Fig. 2b was shifted to 1634 in Fig. 2c because of interactions between naringenin and acrylamide monomer. The obtaining findings show that the naringenin molecule was successfully imprinted into Nar-ICM structures when compared to non-ICM ones.
The contact angle value gives information about the hydrophilicity of a surface. Surfaces with contact angles between 0 and 90° show hydrophilic character. The surface hydrophilicity decreases as the contact angle approaches 90°, the surface shows hydrophobic character over 90°. Contact angle measurements of Nar-ICMs and non-ICMs are given in Fig. 3. As seen in Fig. 3a and Fig. 3b, contact angle values of 71.8° and 58.5° were obtained for Nar-ICMs and non-ICMs, respectively. Here, the contact angle of the non-ICMs increased by 22.7%. This can be explained by the aromatic groups on the naringenin molecules imprinted on the polymeric surface.
Scanning electron microscope (SEM) images of Nar-ICMs and non-ICMs taken at different zooms are given in Fig. 3. When the SEM images of Nar-ICMs (Fig. 3b-i and ii) and non-ICMs (Fig. 3b-iii and iv) are examined, it is seen that Nar-ICMs have a porous structure because of imprinting of molecules while the non-ICMs have a relatively smooth structure. The porous morphological structures of Nar-ICMs increase the adsorption capacity by upsizing the surface area in favour of imprinted membranes.
Naringenin adsorption studies from aqueous solution
Effect of pH on naringenin adsorption to Nar-ICMs
While investigating the effect of medium pH on naringenin adsorption to Nar-ICMs, the buffer type was kept constant (0.05 M acetate buffer) and studied at four different pHs. Results were given in Fig. 4.
As seen in Fig. 4a, while the maximum adsorption was observed at pH 5, there are no sharp decreases in the adsorption values to the right and left of this pH, but slight decreases are observed. This can be explained by the absence of active pH sensitive groups in the naringenin molecule.
Effect of temperature on naringenin adsorption to Nar-ICMs
The effect of medium temperature on naringenin adsorption to Nar-ICMs was studied, and the obtained results are given in Fig. 4b. In a typical adsorption study, the temperature parameter can also provide us some information about the interaction parameters (e.g., hydrophobic, ionic) that are dominant in adsorption process. In this study, the maximum adsorption occurred at 20 °C while it dropped away more as the temperature increased. The reason can be interpreted with physical interactions where ionic/hydrogen bonds are dominant in the adsorption process [23].
Effects of time and concentration on naringenin adsorption to Nar-ICMs
The effects of time and concentration on the adsorption of naringenin to Nar-ICMs are given in Fig. 5.
As seen in Fig. 5, although it is not very common at low concentrations, as the concentration increases, the balance against time becomes evident in adsorption. It should be noted that the adsorption equilibrium is reached in a short time such as 20 min even at low initial concentration. Working of Nar-ICMs at low concentrations and in a short time means that these membranes can also be used for industrial purposes with low cost.
Effect of concentration on adsorption and determination of binding parameters
It is an important parameter to determine the binding capacity and affinity of the synthesized adsorbents for purification operations. To this end, the adsorption isotherms of naringenin to Nar-ICMs and non-ICMs are performed and given as Fig. 6.
For the binding capacity, the membranes were interacted with solutions including naringenin in the range of 0.1–3.0 mg/mL, and the maximum adsorption capacities were plotted as seen in Fig. 6a. As can be seen here, the adsorption capacity increases with increasing naringenin concentration. The active binding vacancies in the imprinted zones are being saturated with about 66.5 mg/g at an initial naringenin concentration of about 2 mg/mL. Considering that 78.6 mg of naringenin is used per gram of polymer, it is seen that Nar-ICMs work approximately 85% effectively with a value of 66.5 mg/g. This can be explained by the fact that cryogel membranes allow mass transfer without diffusion restriction due to their macroporous structure. The adsorption amount of non-specific naringenin on non-ICMs was 14 mg/g.
For determination of the binding constants of Nar-ICMs, a Langmuir type isotherm [24], which describes equilibrium reactions between the binding sites of an adsorbent and bound molecules, was used. Scatchard analysis [25] (Eq. 7) derived from Langmuir’s equation was evaluated to examine the binding behaviour of naringenin to Nar-ICMs.
The straight-line plot of equation q*/C* against q* in Eq. 7 indicates an independent interaction between the binding molecules and the binding sites. Here, qm and Kd give the maximum naringenin adsorption (mmol/g) and dissociation constant of naringenin in equilibrium (mM), which can be predicted from the slop and the intercept, respectively. Qe and Ce are naringenin amount bound to Nar-ICMs (mmol/g), and naringenin concentration (mM), respectively. The Scatchard plot for the adsorption of naringenin to Nar-ICMs is given in Fig. 6b.
As seen in Fig. 6b, instead of a single line, two linear equations with high and low-affinity regions appear in the plot. From these two linear lines, it can be concluded that Nar-ICMs have heterogeneous binding sites with high and low affinity for naringenin molecules. While the qm and Kd values in the high-affinity binding sites are 0.126 mmol/g (34.4 mg/g) and 0.16 mM (1.6⨯10–4 M), respectively, the qm and Kd values in the low-affinity binding sites are 0.328 mmol/g (89.4 mg/g) and 2.56 mM (2.56⨯10–3 M). The high domain affinity for naringenin molecules appears to be approximately 15 times greater than the low domain affinity. In addition, for the interaction between the ideal adsorbate-adsorbent, it is preferred that the Kd become small and between 10–4 and 10–8 M (Gurbuz et al., 2016). Considering the obtained Kd value of 1.63⨯10–4 M and other results, it can be concluded that naringenin imprinted membranes have a high affinity for naringenin molecules at low concentrations.
Selectivity studies
Wavelength scanning was performed between 200 and 400 nm for competitor molecules, and 212 nm for gallic acid and 315 nm for caffeic acid were determined as optimal wavelengths. Three statistical values were calculated to evaluate the selectivity studies. These values are KD (distribution coefficient), k (selectivity coefficient) and k′ (relative selectivity coefficient). Here, it should be emphasized that the molecularly imprinted polymer prepared does not have any selectivity if k′ is equal to 1. Statistical values calculated in the light of this information are given in Table S1 (Supplementary File) while the chemical structures of these three molecules are presented in Fig. S1 (Supplementary File).
As can be seen from the chemical structures of template molecules and competitor agents, high KD values of gallic and caffeic acid molecules across to naringenin are not a surprise. Because gallic and caffeic acid molecules are smaller in size compared to naringenin. Due to the size difference, these competitor molecules can easily enter the 3D spaces of naringenin. However, since interaction is not specific, competitor agents can be removed by washing. When we focus on the numerical values in Table 1, especially if we pay attention to the k′ value, we can see that the relative selectivity (k′) of the Nar-ICMs against gallic acid is 1.99 times and against caffeic acid 2.26 times. These values indicate that the naringenin imprinted membrane has an approximate twofold selectivity even against competitor agents with a relatively smaller molecular volume than itself.
Desorption and reusability studies
For the effective desorption of naringenin from Nar-ICMs to create suitable 3D empty zones to purify naringenin molecules from the media, it is very important to remove as much as possible template molecules, which entered the structure during the polymerization period, without disrupting the three-dimensional structure of the polymeric membrane [26]. For this aim, Nar-ICMs were interacted with different solutions at different time intervals, and the results were given in Fig. 7. When the molecular structure of naringenin, given in Fig. S1, is examined, aromatic groups with hydrophobic properties are also seen in addition to the hydrophilic groups in the structure. Although hydrophilic structures are the dominant groups in the specific interaction between naringenin and the membrane, it is possible that the hydrophobic groups on the molecule have also non-specific interactions with the membrane. Based on this argument, solutions with different polarities were used for desorption. As can be seen in Fig. 7a, deionized DI water and NaCl solution did not show much success in desorption while methanol and ethanol molecules carrying apolar groups in their structure together with the polar group showed higher desorption performance. Among these molecules, especially ethanol, which has a higher apolar property compared to methanol, showed a desorption property of over 90%.
In order to demonstrate the stability and reusability of Nar-ICMs, the adsorption–desorption cycle was repeated six times using the same membrane, and the results were given in Fig. 7b. Without regeneration of the membranes, only desorption was performed after each adsorption cycle (in case of serious adsorption loss in adsorbents, a regeneration process is performed with 50 mM NaOH solution). At the end of six adsorption–desorption cycles, only a 12% reduction in adsorption capacity was seen.
Naringenin adsorption studies from natural source
Adsorption studies of naringenin were carried out with the liquid part of OJ obtained by squeezing fresh fruit. After removing the pulp by centrifugation at 1000 rpm, three different solutions of OJ (e.g., pure OJ, 1/2 and 1/10 diluted fresh solutions) were prepared. While the maximum adsorption for naringenin occurred as 843 ± 8 µg/g in 1/2 diluted OJ, naringenin adsorption for pure OJ and 1/10 diluted OJ came true as 173 ± 5 and 185 ± 7 to Nar-ICMs, respectively. Although the adsorption of naringenin from the aqueous medium increased as the concentration raised up, such a situation was not observed in the natural environment. It is noted that non-specific naringenin adsorption on non-ICMs was a negligible amount. This can be interpreted as the density of molecules other than naringenin in the natural environment prevents naringenin from approaching 3D recognition regions.
Conclusion
Although lots of studies on the determination and separation of naringenin are available in the literature, research on naringenin purification have not been found [27, 28]. However, both MIP and other chromatographic-based purification studies of naringin have been carried out by researchers [29, 30].
In this study, naringenin imprinted cryogel membranes were synthesized, and investigated for some chromatographic features for naringenin purification from the natural environment. While AAm and Nar were used as functional monomer and template with the ratio of 1/3 (Nar/AAm), HEMA and MAAm were used as co-monomer and cross-linker, respectively. Here, gallic and caffeic acid were used as competitor agents, and prepared naringenin imprinted cryogel membranes were found to be about two times more selective against these molecules. The maximum adsorption capacities of Nar-ICMs and non-ICMs for naringenin were found to be 66.5 and 14 mg/g, respectively at an initial concentration of 2 mg/mL. The number of reuses of a polymeric sorbent synthesized for purification purposes is very important for the economy of the process. Here, the synthesized membranes for the purification of naringenin were used repeatedly without any significant reduction in their capacity. In conclusion, in this study, the use of new synthesized cryogel-based naringenin imprinted membranes has been demonstrated as a selective and efficient purification sorbent for naringenin.
References
Stabrauskiene J, Kopustinskiene DM, Lazauskas R, Bernatoniene J (2022) Naringin and naringenin: their mechanisms of action and the potential anticancer activities. Biomedicines 10:1686
Patel K, Singh GK, Patel DK (2018) A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med 24:551–560
Sant A, Ahmad I, Bhatia S (2022) Extraction and hydrolysis of naringin from citrus fruit peels. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing, p 12031
Noori S, Tavirani MR, Deravi N et al (2020) Naringenin enhances the anti-cancer effect of cyclophosphamide against MDA-MB-231 breast cancer cells via targeting the STAT3 signaling pathway. Iran J Pharm Res IJPR 19:122
Chang H, Chang Y, Lai S et al (2017) Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and-9. Exp Ther Med 13:739–744
Choudhury R, Chowrimootoo G, Srai K et al (1999) Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem Biophys Res Commun 265:410–415
Haupt K, Mosbach K (2000) Molecularly imprinted polymers and their use in biomimetic sensors. Chem Rev 100:2495–2504. https://doi.org/10.1021/cr990099w
Acet Ö, Noma SAA, Acet BÖ et al (2023) A rational approach for 3D recognition and removal of L-asparagine via molecularly imprinted membranes. J Pharm Biomed Anal 226:115250
Inanan T, Tüzmen N, Akgöl S, Denizli A (2016) Selective cholesterol adsorption by molecular imprinted polymeric nanospheres and application to GIMS. Int J Biol Macromol 92:451–460
Arabi M, Ostovan A, Li J et al (2021) Molecular imprinting: green perspectives and strategies. Adv Mater 33:2100543
BelBruno JJ (2018) Molecularly imprinted polymers. Chem Rev 119:94–119
Acet Ö, Odabaşı M (2022) Detection of N-hexanoyl-L-homoserine lactone via MIP-based QCM sensor: preparation and characterization. Polym Bull 1–18
Dikici E, Acet BÖ, Acet Ö, Odabaşi M (2023) “Lab-on-pol” colormatic sensor platforms: melamine detection with color change on melamine imprinted membranes. Microchem J 188:108468
Karasu T, Özgür E, Uzun L (2023) MIP-on-a-Chip: artificial receptors on microfluidic platforms for biomedical applications. J Pharm Biomed Anal 226:115257
Wackerlig J, Schirhagl R (2016) Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: a review. Anal Chem 88:250–261
Ertürk G, Mattiasson B (2014) Cryogels-versatile tools in bioseparation. J Chromatogr A 1357:24–35
Lozinsky VI, Galaev IY, Plieva FM et al (2003) Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol 21:445–451. https://doi.org/10.1016/j.tibtech.2003.08.002
Baydemir G, Bereli N, Andaç M et al (2009) Bilirubin recognition via molecularly imprinted supermacroporous cryogels. Colloids Surfaces B Biointerfaces 68:33–38
Karaduman AB, Çetin K (2023) Molecularly imprinted cryogels for the selective adsorption of salicylic acid. Appl Biochem Biotechnol 195:1877–1887
Ceylan Ş, Odabaşı M (2013) Novel adsorbent for DNA adsorption: Fe 3+ -attached sporopollenin particles embedded composite cryogels. Artif Cells, Nanomedicine, Biotechnol 41:376–383. https://doi.org/10.3109/21691401.2012.759125
Odabaşı M, Uzun L, Baydemir G et al (2018) Cholesterol imprinted composite membranes for selective cholesterol recognition from intestinal mimicking solution. Colloids Surfaces B Biointerfaces 163:266–274. https://doi.org/10.1016/j.colsurfb.2017.12.033
Acet Ö, Önal B, Sanz R et al (2019) Preparation of a new chromatographic media and assessment of some kinetic and interaction parameters for lysozyme. J Mol Liq 276:480–487. https://doi.org/10.1016/j.molliq.2018.12.037
Feng X, Wu T, Yu B et al (2017) Hydrophilic surface molecularly imprinted naringin prepared via reverse atom transfer radical polymerization with excellent recognition ability in a pure aqueous phase. RSC Adv 7:28082–28091
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Scatchard G (1949) The attractions of proteins for small molecules and ions. Ann N Y Acad Sci 51:660–672
Cömert ŞC, Özgür E, Uzun L, Odabaşı M (2022) The creation of selective imprinted cavities on quartz crystal microbalance electrode for the detection of melamine in milk sample. Food Chem 372:131254
Rebello CJ, Beyl RA, Lertora JJL et al (2020) Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes, Obes Metab 22:91–98
Cordenonsi LM, Bromberger NG, Raffin RP, Scherman EE (2016) Simultaneous separation and sensitive detection of naringin and naringenin in nanoparticles by chromatographic method indicating stability and photodegradation kinetics. Biomed Chromatogr 30:155–162
Trotta F, Drioli E, Baggiani C, Lacopo D (2002) Molecular imprinted polymeric membrane for naringin recognition. J Memb Sci 201:77–84
Jiang X, Zhou J, Zhou C (2006) Study on adsorption and separation of naringin with macroporous resin. Front Chem China 1:77–81
Acknowledgements
This work was supported by The Scientific and Technological Research Council of Turkey (Grant Number: 121Z173). Authors gratefully acknowledge for use of the services and facilities of Scientific and Technological Application and Research Center of Aksaray University (ASUBTAM).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Demirtaş, E., Odabaşı, M. Preparation and application of naringenin imprinted cryogel membranes for selectively separation of naringenin from natural environment. Polym. Bull. 81, 11795–11811 (2024). https://doi.org/10.1007/s00289-024-05266-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05266-1