Abstract
In this study, the transport performance of acetaminophen (paracetamol), which is most commonly prescribed and used for humans and animals and whose wastes are known to have toxic effects on the environment and some living organisms, was investigated using zinc oxide (ZnO)-reinforced polymer membranes. In this study, nanoparticle-containing polymer membranes were prepared from cellulose triacetate in dichloromethane and ZnO nanoparticles were synthesized to impart adsorption properties to the membrane in a single step, enabling adsorption and filtration to improve the removal of low molecular weight micropollutants that are poorly retained by conventional polymer membranes and by enabling re-release. Our membrane was prepared by phase inversion method by doping with cellulose triacetate (CTA) solution. Parameters such as carrier concentration, mixing rate, transport time, acceptor and feed phase concentrations were studied to determine the optimal conditions for the transport experiments of paracetamol (PARA). The presence of acid in the acceptor phase converted the hydrophilic part of paracetamol, which allowed the transport of PARA. The calculated flux values for different PARA concentrations ranged from (0.64) × 10−8 to (1.8) × 10−8 mol/(cm2s). Under optimal conditions, a transport efficiency of 84% was obtained for PARA with a CTA/ZnO polymer membrane. The obtained membranes can be used in wastewater treatment, recovery of pharmacological products from pharmaceutical industry waste, re-evaluation of hospital waste, etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the rapid population growth in the world and the industrial development of countries, the excessive amount of domestic and industrial wastewater is increasing, causing health, environmental and economic problems. One of the reasons for the increasing pollution is that most countries discharge their wastewater into the environment and the sea without treatment. Nowadays, the existence of pharmaceuticals is curative [1, 2]. These substances are manufactured to remain permanently in the human body after administration to prolong their effects. Therefore, these pharmaceuticals enter wastewater and the environment and pose a serious threat to our aquatic environment and organisms. Some pharmaceutical molecules can have lethal consequences and are of concern due to their biological effects. For example, PARA has toxic effects on some marine animals. PARA is the most commonly prescribed and used analgesic for humans and animals and has been frequently detected in wastewater. Therefore, it is important to remove it from the aquatic environment. In addition, acetaminophen is highly soluble in water, making it difficult to remove in wastewater treatment plants, and has been found in surface waters and groundwater [3,4,5]. Some properties of PARA are listed in Table 1.
In fact, it is very important to reuse industrial wastes, recover valuable molecules and develop new effective separation processes to make this possible. Although adsorption seems to be one of the most effective methods for water purification of various materials and pharmaceutical products, its widespread application is limited due to its high cost [6, 7]. Membranes are known as a technology that can be applied in any environment that is constantly evolving. Moreover, this method is simple, easy to develop, reliable, applicable on a large scale and inexpensive. The basic polymer used for the mechanical strength of the membranes is of great importance. Although a variety of base polymers are used in many fields, it is surprising that the two most important polymers are polyvinyl chloride (PVC) and CTA in researches on polymer including membranes (PIMs). It is obtained by using an extractant, plasticizer and a basic polymer such as CTA in the structure to provide flexibility and form a stable film in PIMs. Plasticizers are used in the production of PIM to make the membrane flexible and soft and to increase its flow rate. However, excessive use causes the membrane to lose its flexibility and rupture. Moreover, plasticizers provide this increase in two ways, by getting between the polymer molecules. First, they neutralize the polymer’s polar groups with their own polar groups, and second, they reduce the strength of the intermolecular forces by simply increasing the distance between the polymer molecules [8].
Polymeric membranes are structurally very susceptible to chemical and physical degradation and remain constant in their specific properties due to the nature of the material used. To avoid this, new polymer-based membranes can be developed to meet the requirements of many practical applications by combining polymer membranes with inorganic materials [9,10,11,12]. The addition of inorganic materials to the polymer improves some physicochemical properties of the membranes such as mechanical stability, porosity, pore size and hydrophilicity. The materials used in the preparation of membranes should be carefully selected. When using such materials, especially in the pharmaceutical industry, it is important to minimize the health effects of cellulose-based polymers. Used as a membrane material, CTA stands out for its high hydrolysis resistance, high salt resistance and durability. CTA is a compound of acetate ester and cellulose. It is widely used as the main source for the production of some films. To overcome the disadvantage of limited solubility of CTA in common solvents and to improve CTA membranes, coating, doping and modification methods have been applied to form a covalent bond with CTA. In the literature, hydrophilic monomers such as polymethyl acrylate and poly(butyl acrylate) are commonly used for surface modification of CTA membranes. In addition, as shown in some studies, it may be necessary to increase the hydrophilicity of the surface of the CTA membrane by various methods. For example, a thin hydrophilic polymer layer can be formed by immersing the membrane in a polyamide solution to increase the performance of the membrane [13].
Nowadays, a modified polymerization method is used in which trimesoyl chloride (TMC) is reacted after dissolving CTA on the CTA membrane. In this way, covalent bonds are formed between the support material and the active layer, increasing the performance of the membrane and stabilizing its structure. Membranes need to be developed that eliminate these drawbacks of membranes and increase their performance and efficiency. For this reason, the addition of composites, inorganic and/or organic materials with different properties to membranes has recently become a common method [14]. In particular, the selection of inorganic additives (nanoparticles) at the nanoscale and the use of nanocomposite hybrid materials in the membrane structure have added a new dimension to membrane technology [15]. Nanoparticles and composite structures have high stability, chemical and biological resistance, unique physicochemical properties, ability to function in a wide pH range, easy functionalization, antibacterial properties, protection against fouling, etc.. Nanoparticles and some composite materials are added to the membrane structure to take advantage of their properties and eliminate the disadvantage of the hydrophobic polymer structure of the membrane. The production and development of a new generation of membranes based on nanomaterials has become one of the most important priorities to meet the requirements of domestic and industrial wastewater treatment. Recently, much attention has been paid to the doping of hydrophilic polymers with metal oxide nanoparticles. In this way, the use of nanoparticles in membrane production has increased due to their superior physicochemical properties to prevent membrane fouling [16,17,18,19].
We have found that the most common problem in membrane applications is contamination, and studies in the literature show that nanoparticles are very useful in solving this problem [20]. There are numerous studies in the literature using nanoparticles of silicon dioxide (SiO2), titanium (TiO2), aluminum (Al2O3), palladium (Pd), silver (Ag), gold (Au), iron (FeO, Fe3O4) [20] and hybrid structures [21, 22]. ZnO nanoparticles, like titanium nanoparticles, are the most important ingredients in creams, coatings, and body lotions because they block solar radiation and have antibacterial properties. There is no study in the literature on the purification of an active pharmaceutical ingredient such as paracetamol from wastewater by a ZnO-modified CTA composite membrane. In this study, CTA/ZnO composite membranes were prepared by phase inversion and their chemical composition, membrane morphology and hydrophilicity were investigated. Then, these membranes were used for PARA transport experiments with aqueous PARA solutions. Due to the ease of implementation in purification systems and the low-cost fabrication of the CTA/ZnO polymer membrane system, an avenue for simple structured membrane studies with commercialization potential may open up.
Experimental
Materials
The chemicals used in this study, such as cellulose triacetate, ZnCl2(analytically pure), NH4OH(%25), CON2H4(analytically pure), CH2Cl2, and CH3CH2OH(extra pure), were purchased from Merck. Paracetamol was purchased from Lianyungag Kangle Pharmaceutical Co. Ltd. All solutions were prepared using ultrapure water (UPW) during the experiments.
Synthesis of zinc oxide nanoparticles
In the synthesis of ZnO nanoparticles, the synthesis procedure of Akin and Ersoz [23] was used. 1/4 by weight of ZnCl2/urea was dissolved in 100 ml of UPW with continuous stirring (300 rpm), and NH4OH solution was added dropwise until the pH of the medium was about 10. Then the solution was heated to 200 °C in a Teflon autoclave and kept there for 8 h. The white precipitates were filtered by successive washing with ethanol and UPW and dried at 30 °C. Finally, calcination was carried out at 400 °C for 6 h [23].
Preparation of CTA membrane
Figure 1 represents the schematic diagram of the preparation of the CTA membrane. Prior to the preparation of the CTA membranes, ZnO nanoparticles were stored overnight in an oven at 105 °C to remove adsorbed water from the obtained the ZnO nanoparticles. CTA membranes doped with ZnO nanoparticles were prepared by the phase inversion method. 400 mg of CTA was taken and 40 mL of dichloromethane (DCM) was added at room temperature. Then, 0.6 mL of 2-nitro phenyl octyl ether (2-NPOE) in 5 mL of DCM was added to the mixture. ZnO nanoparticles in various ratios (ranging from 0.1 to 3%) were dispersed in 5 mL of DCM and added to the CTA solution, and the mixture was stirred for 1 h. The CTA-ZnO was kept in membranes and distilled water and separated from the petri dish [24, 25].
Membrane surface characterization
To characterize the composite membranes, surface morphology was studied using a ATR FTIR spectrometer (Perkin Elmer 100 FTIR), contact angle measurements using KSV CAM 200 and membrane surface images were taken with a Zeiss Primovert microscope at 40X objective. Membrane surface, cross-section images and EDX analysis taken with a scanning electron microscope (SEM) EVO-LS 10 (Carl Zeiss, Germany).
Transport experiments
Transport experiments were performed using a membrane cell system consisting of two separable Teflon chambers with a capacity of 50 ml. The membranes obtained were placed between the rubber seals by squeezing them together to prevent leakage between the chambers. The mixing speed of the two chambers was kept constant at 350 rpm during each transport run. Measurements of all PARA samples were taken at 234 nm using a UV spectrophotometer. As a result of the transport studies, we intend to develop a commercially viable disposable prototype syringe filter system with the best performing membrane. We also believe it will pioneer the development of new systems that allow purification without leaving too much harmful waste in the environment.
Results and discussion
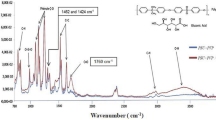
The FTIR spectrum of the obtained membranes was taken and given in Fig. 2. Examination of the FTIR spectra of CTA and ZnO nanoparticles reveals a characteristic Zn–O vibrational band around 545 cm−1, confirming that synthesis of ZnO nanoparticles has occurred [26]. In pure ZnO nanoparticles, broad absorption peaks are observed at the peak values of 3450 and 1630 cm−1. These are due to the O–H stress of the absorbed water due to the large surface-to-volume ratio of the nanoparticles and CO2. In CTA, weak tensile vibrations around 2930 cm−1 were attributed to aliphatic C–H groups. It shows the absorption bands of carbonyl group around 1740 cm−1. The absorption band at 1375 cm−1 is due to C–H decays in methyl groups. In addition, two absorption bands at 1032 and 1205 cm−1 are due to the stretching mode of the C–O group.
The photographic image and light microscope images of the obtained membranes are shown in Fig. 3. As seen in the light microscopy images (Fig. 3), the CTA composite membrane had an in homogeneous surface with air bubbles on the surface, while the addition of ZnO nanoparticles gave the surface a homogeneous appearance.
The synthesized membrane surfaces were examined with SEM. Figure 4 shows the SEM images of the fabricated membranes. The more homogeneous and smooth appearance of the CTA membrane surface was significantly changed by the introduction of ZnO nanoparticles into the membrane structure. It is believed that the surface undulations and plate-shaped accumulations result from the accumulation of ZnO nanoparticles at certain locations during phase inversion in the CTA solution. A similar situation was found in the study of Balta et al. [27]. Additionally, when the cross-sectional images of the obtained membranes were examined, the thickness of the CTA and CTA-ZnO membranes was measured as 52.3 µm and 45.4 µm, respectively. The arrangement of the layers is clearly visible in the cross-sectional image of the CTA membrane. However, with the addition of ZnO to the membrane structure, it is seen that the structure becomes completely bulk. The EDX analysis shows the presence of ZnO nanoparticles in the membrane structure and a homogeneous distribution. In the EDX analysis, it was observed that the membrane contents contained approximately 65% carbon and 35% oxygen for the CTA membrane. It was determined that the CTA/ZnO membrane contained approximately 30% carbon, 60% oxygen and 10% zinc. The results obtained are given in detail in Table 2.
As shown in Fig. 5, contact angle measurements were performed to obtain information about the hydrophilicity of the surfaces of CTA and CTA/ZnO membranes [28,29,30]. According to the measurement results repeated three times at room temperature, the measured values of CTA and CTA/ZnO membranes were 61 ± 1° and 63 ± 1°, respectively. For polymer membranes, the contact angle value increases or decreases depending on the material added to the membrane structure. The increase in contact angle provides information about the wettability of the membrane surface. An increase in the contact angle means less adhesion of the liquid to the surface. The reason why the contact angle of the CTA membrane is lower than that of the CTA/ZnO membrane is because ZnO nanoparticles are incorporated into the CTA polymer.
The effect of paracetamol concentration on feed phase
In the transport study, transport experiments were performed with four different PARA concentrations ranging from 0.005 to 0.1% to investigate the effects of the concentration of PARA in the feed solution on transport. The results are shown in Fig. 6. The effects of the concentration of PARA on transport were evaluated using the flux values in Eq. (1). As the concentration of PARA increased, the transfer rate decreased from 84 to 54% after 24 h of transport. The calculated flux values for different concentrations of PARA ranged from (0.64) × 10−8 to (1.8) × 10−8 mol/(cm2s). The results show that a high concentration leads to a higher flux and an increase in the concentration of PARA in the feed phase causes a decrease in the transport rate [23].
Effect of stripping phase concentration
Studies were performed with 0.1 to 3 M HCl solution to investigate the effects of acceptor phase concentration on the transport of PARA. The transport of PARA increased with increasing HCl concentration, as shown in Fig. 7. The presence of acid in the acceptor phase converted the hydrophilic part of paracetamol, which allowed the transport of PARA. The transport of PARA showed a parabolic increase up to a concentration of 2 M, while a slight decrease was observed at an acid concentration of 3 M HCl.
The effect of carrier concentration
The efficiency of transport in polymer membranes prepared by the phase inversion method depends on the functional groups of the polymer source. However, the material added to the polymer, namely the carrier molecule, is crucial. The concentration of the carrier molecule ZnO nanoparticles in the membrane structure has a significant effect on the transport of paracetamol across the membrane. The effect of the amount of ZnO nanoparticles on the transport of PARA was studied at four different carrier concentrations ranging from 0.1 to 3%. Figure 8 shows the effect of the ratio of doped ZnO nanoparticles on the transport of PARA in the CTA/ZnO membrane. The experiments showed that the transport rate increased when the ZnO content was increased to 1%, while a decrease of 3% was observed. In the literature, similar results were observed in transport studies of dyes, metals and some organic molecules with different supports [31]. It is suggested that this decrease is due to the interaction PARA /ZnO at the interface between the feed solution and the membrane, which becomes saturated after a certain time at the membrane surface, i.e., causes steric hindrance. In order to eliminate this decrease in such processes, in the literature, changes are usually made to the porcelain parameters or the membrane surface is affected with various applications (electric current, vibration, etc.). However, in this type of research, the membranes produced are usually disposable membranes, as they are preferred in accordance with the modules designed for practical applications [23].
The effect of transport time and stirring speed
To investigate the performance of the membranes during 24-h transport, a series of experimental setups were fabricated and the optimal transport time was determined by measuring the amount of PARA in both the feeding and acceptor phases at specific time intervals; the results are shown in Fig. 9. The results show that the transport process lasts up to 8 h and is stable after 8 h. From Fig. 10, it can be seen that as the mixing speed increases, the transport ratio increases in parallel up to a certain point. However, with the increase of mixing speed after 350 rpm, the maximum of transport, a significant decrease of transport ratio is observed. The reason for this is the turbulence effect caused by the mixing speed, for which there are numerous examples in the literature [32, 33]. All other experiments were carried out at 350 rpm.
Conclusions
In this study, the transport performance of PARA in a versatile polymer membrane doped with ZnO was investigated by the phase inversion method. To determine the optimal conditions for transport experiments with the obtained membranes, the effects of variables such as time, ratio of ZnO nanoparticles as carriers, PARA concentration, mixing rate and acceptor phase concentration were investigated. The results showed that the incorporation of ZnO into the CTA polymer matrix was successful. Thus, the fabrication of a polymer-containing membrane doped with ZnO nanoparticles was possible. Based on the experiments performed to determine the optimal conditions in the transport experiments of PARA, the maximum transport of PARA was achieved under the following experimental conditions: membrane prepared with 1.0% ZnO nanoparticles, stirring speed 350 rpm, transport time 10 h and acceptor phase concentration of 1 M HCl. On the other hand, the transport fraction decreased when the concentration of PARA in the feed phase was increased from a certain point. SEM Results showed that the uniform and smooth appearance of the CTA membrane surface was significantly changed by the addition of ZnO nanoparticles to the membrane structure.
The calculated flux values for different PARA concentrations ranged from (0.64) × 10−8 to (1.8) × 10−8 mol/(cm2s). The results show that a high concentration leads to a higher flux and an increase in the concentration of PARA in the feed phase causes a decrease in the transport rate. The results of transport experiments showed that the transport rate increased when the amount of ZnO was increased to 1%, while a decrease of 3% was observed. The performance test results showed that the transport process for the obtained membranes lasted up to 8 h and was stable after 8 h. Under optimal conditions, a transport efficiency of 84% was obtained for PARA with a CTA/ZnO polymer membrane. Our polymer-containing membranes doped with ZnO nanoparticles can be used in wastewater treatment, recovery of pharmacological products, re-evaluation of hospital waste, etc.
References
Lin AYC, Wang XH, Lin CF (2010) Impact of wastewaters and hospital effluents on the occurrence of controlled substances in surface waters. Chemosphere 81:562–570. https://doi.org/10.1016/j.chemosphere.2010.08
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment. Sci Total Environ 429:123–155. https://doi.org/10.1016/j.scitotenv.2012.04.028
Marta M, Strankowska J, Kwela J, Strankowski M, Józefowicz M (2020) Transport of paracetamol in swellable and relaxing polyurethane nanocomposite hydrogels. Polym Bull 77:483–499. https://doi.org/10.1007/s00289-019-02755-6
Abid S, Hussain T, Nazir A, Zahir A, Khenoussi N (2019) A novel double-layered polymeric nanofiber-based dressing with controlled drug delivery for pain management in burn wounds. Polym Bull 76:6387–6411. https://doi.org/10.1007/s00289-019-02727-w
Alpaslan D, Turan A, Dudu TE, Aktas N (2023) Evaluation of particle biosynthesis, p (Okra) particle bioactivity, and drug release properties using Abelmoschus esculentus (okra) plant extract. Polym Bull 7:1–16. https://doi.org/10.1007/s00289-023-04923-1
Beninati S, Semerato D, Mastragostino M (2008) Adsorption of paracetamol and acetylsalicylic acid onto commercial activated carbons. Adsorpt Sci Technol 26:721–773. https://doi.org/10.1260/026361708788251349
Ruiz B, Cabrita I, Mestre AS, Parra JB, Pires J, Carvalho AP, Ania CO (2010) Surface heterogeneity effects of activated carbons on the kinetics of paracetamol removal from aqueous solution. Appl Surf Sci 256:5171–5175. https://doi.org/10.1016/j.apsusc.2009.12.086
Sears JK, Darby JR (1982) Technology of plasticizers. John Wiley & Sons, New York, p 1174
Arthanareeswaran G, Devi TKS, Mohan D (2009) Development, characterization and separation performance of organic–inorganic membranes. Part II. Effect of additives. Sep Purif Technol 67:271–281. https://doi.org/10.1016/j.seppur.2009.03.037
Mansouri J, Harrisson S, Chen V (2010) Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J Mater Chem 20:4567–4586. https://doi.org/10.1039/b926440j
Yang HC, Hou J, Chen V, Xu ZK (2013) Surface and interface engineering for organic inorganic composite membranes. J Mater Chem A 25:1–15
Yin J, Baolin D (2015) Polymer-matrix nanocomposite membranes for water treatment. J Membr Sci 479:256–275. https://doi.org/10.1016/j.memsci.2014.11.019
Alsvik IL, Zodrow KR, Elimelech M, Hägg MB (2013) Polyamide formation on a cellulose triacetate support for osmotic membranes: effect of linking molecules on membrane performance. Desalination 312:2–9. https://doi.org/10.1016/j.desal.2012.09.019
Akın İ (2015) Preparation of nanoparticle containing composite membrane and application areas. Ph.D. Thesis, Selcuk University, Institute of Science and Technology, Konya, pp 1–13
Saleh TA, Gupta VK (2016) Nanomaterial and polymer membranes: Synthesis, characterization and applications. Elsevier, Amsterdam, pp 1–272
Li JF, Xu ZL, Yang H, Yu LY, Liu M (2009) Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl Surf Sci 255:4725–4732. https://doi.org/10.1016/j.apsusc.2008.07.139
Cortalezzi MM, Rose J, Barron AR, Wiesner MR (2002) Characteristics of ultrafiltration ceramic membranes derived from alumoxane nanoparticles. J Membr Sci 205:33–43. https://doi.org/10.1016/s0376-7388(02)00049-2
Soroko I, Livingston A (2009) Impact of TiO2 nanoparticles on morphology and performance of crosslinked polyimide organic solvent nanofiltration (OSN) membranes. J Membr Sci 343:189–198. https://doi.org/10.1016/j.memsci.2009.07.026
Sueraya AZ, Rezaur MD, Devagi R, Anwar KK, Anthonette MS, Al-Khalid J, Khusairy OM, Uddin BC (2023) A comprehensive review on nanocellulose-based membranes: methods, mechanism, and applications in wastewater treatment. Polym Bull. https://doi.org/10.1007/s00289-023-05084-x
Kim J, Van der Bruggen B (2010) The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. Environ Pollut 158:2335–2349. https://doi.org/10.1016/j.envpol.2010.03.024
Babu A, Somesh TE, Ani Dechamma CD, Hemavathi AB, Kakarla RR, Kulkarni RV, Raghu AV (2023) Ternary structured magnesium cobalt oxide/graphene/polycarbazole nanohybrids for high performance electrochemical supercapacitors. Mater Sci Energy Technol 6:399–408. https://doi.org/10.1016/j.mset.2023.04.002
Shivaprasad N, Veena MG, Madhukar BS, Kavya R, Sarath K, Vanga PR, Babu GSD, Sachith BM, Raghu AV (2024) Highly flexible, green luminescent down converting and hydrophobic 0-D cesium lead bromide (Cs4PbBr6)/ poly (vinylidene difluoride) polymer nanocomposites for photonics and display applications. Inorg Chem Commun 159:111761. https://doi.org/10.1016/j.inoche.2023.111761
Akın I, Ersoz M (2016) Preparation and characterization of CTA/m-ZnO composite membrane for transport of Rhodamine B. Desalin Water Treat 57:3037–3047. https://doi.org/10.1080/19443994.2014.980327
Kebiche-Senhadji O, Mansouri L, Tingry S, Seta P, Benamor M (2008) Facilitated Cd(II) transport across CTA polymer inclusion membrane using anion (Aliquat 336) and cation (DE2HPA) metal carriers. J Membr Sci 310:438–445. https://doi.org/10.1016/j.memsci.2007.11.015
Yilmaz A, Arslan G, Tor A, Akin I (2011) Selectively facilitated transport of Zn(II) through a novel polymer inclusion membrane containing Cyanex 272 as a carrier reagent. Desalination 277:301–307. https://doi.org/10.1016/j.desal.2011.04.045
Al-Hajry A, Umar A, Hahn YB, Kim DH (2009) Growth, properties and dye-sensitized solar cells–applications of ZnO nanorods grown by low-temperature solution process. Superlattices Microstruct 45:529. https://doi.org/10.1016/j.spmi.2009.02.003
Balta S, Sotto A, Luis P, Benea L, Bruggen BV, Kim J (2012) A new outlook on membrane enhancement with nanoparticles: the alternative of ZnO. J Membr Sci 389:155–161. https://doi.org/10.1016/j.memsci.2011.10.025
Wu CM, Xu TW, Yang WH (2003) Fundamental studies of a new hybrid (inorganic–organic) positively charged membrane: membrane preparation and characterizations. J Membr Sci 216:269–278. https://doi.org/10.1016/s0376-7388(03)00082-6
Bae TH, Kim IC, Tak TM (2006) Preparation and characterization of fouling-resistant TiO2 self-assembled nanocomposite membranes. J Membr Sci 275:1–5. https://doi.org/10.1016/j.memsci.2006.01.023
Alguacil FJ, Coedo AG, Dorado MT, Sastre AM (2001) Uphill permeation of chromium Cr(VI) using Cyanex 921 as ionophore across an immobilized liquid membrane. Hydrometallurgy 61:13–19. https://doi.org/10.1016/s0304-386x(01)00147-5
Mitiche L, Tingry S, Seta P, Sahmoune A (2008) Facilitated transport of copper(II) across supported liquid membrane and polymeric plasticized membrane containing 3-phenyl-4-benzoylisoxazol-5-one as carrier. J Membr Sci 325:605–611. https://doi.org/10.1016/j.memsci.2008.08.021
Salima A, Ounissa K, Lynda M, Mohamed B (2012) Cationic dye (MB) removal using polymer inclusion membrane (PIMs). Procedia Eng 33:38–46. https://doi.org/10.1016/j.proeng.2012.01.1174
Muthuraman G, Teng TT (2009) Use of vegetable oil in supported liquid membrane for the transport of Rhodamine B. Desalination 249:1062–1066. https://doi.org/10.1016/j.desal.2009.05.017
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Selimoglu, F., Akin, I. & Ayhan, M.E. Paracetamol transport by ZnO nanoparticle-doped polymer-containing membrane. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05178-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05178-0