Abstract
Star-shaped SBRs with alkoxy silyl groups in the center of the molecule and amine groups at the polymer chain ends were prepared. The number of arms were varied, and its influence on the silica/silane-filled compound properties was investigated. The polymer–filler interactions and the filler–filler interactions were evaluated from the viscoelastic properties. The flocculation, tensile properties and tanδ at 60 °C, an indicator of rolling resistance, were investigated as well. The polymer–filler interactions were increased in the SBR with a higher number of arms due to the higher amount of amine groups in these SBRs. The Payne effect was slightly increased with a higher number of arms. This indicates a deteriorated micro-dispersion of the silica caused by too many interactions generated by the higher amount of amine groups in SBR. These high interactions due to the amine groups lead to higher tensile moduli. The combination of the higher polymer–filler interactions and the higher Payne effect in SBR with a high number of arms results in the same tanδ compared to the SBR with a low number of arms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the growing interest in the global warming, improving the fuel efficiency of the vehicles is one of the most important targets of the automotive industry. The National Research Council reported that the impact of the rolling resistance of tires on the fuel efficiency is around 10% [1]. As treads are the parts of tires which are directly in contact with the road surface, reducing the hysteresis of the tread compound, a main cause of rolling resistance, became a major target in the market. As silica, the filler of choice for the tire tread, has silanol groups on the surface which causes re-clustering, it is challenging to disperse hydrophilic silica in the hydrophobic rubber matrix. A tread compound containing a silane/silica system was invented in 1992 to solve this issue and lead to an improved rolling resistance as well as wet grip [2]. The silane is bi-functional; the alkoxy silyl group reacts with the silica to reduce the re-clustering tendency and the sulfur part is coupled to the polymer chain to improve the reinforcement. Since then, a lot of studies have been done to further improve the rolling resistance of the silane/silica-filled compounds like optimizing the mixing conditions of silane/silica compounds [3, 4], evaluation of the structure of the silane [5,6,7,8] and investigating the effect of amine on the silanization [9, 10].
Functionalized SBR is another approach to reduce the filler–filler interactions of silica by increasing the polymer–filler interactions. Many functionalized SBRs have been studied [11,12,13,14]. Sone et al. reported the influence of different functional groups on the compound properties [15]. Döring et al. and Hayashi et al. studied functionalized SBRs with functional groups along the chain [16, 17]. However, there are only few studies about the influence of the branching structure of the functionalized SBR on the compound properties [18, 19]. In a previous study [20], it was reported that an amine-functionalized linear SBR did not show any significant improvement neither in Payne effect, the polymer–filler interactions, nor in tanδ. In contrast to this, an alkoxy silyl functionalization of SBR leads to increased polymer–filler interactions, reduced Payne effect, and decreased tanδ at 60 °C, an indicator of rolling resistance. However, the amine groups could increase the polymer–filler interactions resulting in a lower tanδ, when the amount of the amine groups in SBR was increased in a branched structure. These results propose a possibility of a further improvement of the silica compound properties by increasing the amount of the amine groups in SBR.
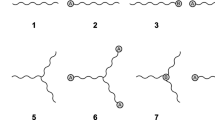
In this study, SBRs with different number of arms were prepared by introducing different functionalization agents (Fig. 1). The influence of the amine groups at the polymer chain ends on the polymer–filler interaction and on the compound properties was investigated as well.
There are a lot of possible interactions and reactions when these polymers are mixed with silica and silane (Fig. 2): the interactions of the amine groups and silica, the reaction between the alkoxy silyl groups and silica, the silica–silane–polymer coupling, and the silica–silane–functional group coupling. Furthermore, the accessibility of the alkoxy silyl groups in the center of the polymer decreases as the number of arms increases. An increase in entanglements with an increasing number of arms also increases the storage modulus at 100% strain. In order to clarify each contribution to the polymer–filler interaction, the compounds were mixed at different temperatures. The factors which influence the polymer–filler interactions most and the impact of each polymer–filler interaction on the silica compound properties are discussed in this study.
The modulus of the filled rubber compound decreases as the strain increases and shows a nonlinear behavior, which is called the “Payne effect” [21]. The different contribution to the modulus is depicted in Fig. 3. This viscoelastic behavior is reviewed by Medalia [22]. The polymer network causes an increased modulus, based on a cross-linked rubber matrix. The hydrodynamic effect is the contribution of the viscosity increase in a liquid by the addition of rigid particles. The in-rubber structure is related to the silica–silane–polymer bonding, the polymer–filler interactions. Filler–filler interaction is the strain-dependent contribution. The filler–filler network which is constituted of physical bonds such as van der Waals forces and hydrogen bonds is partly and finally completely broken down with increasing strain. In the case of the compounds with functionalized SBRs, the polymer–filler interactions caused by the interactions between the functional groups in SBRs and silica increase the modulus. Therefore, the shear modulus is used as an indicator of the polymer–filler interactions to investigate the influence of the functionalized SBRs.
Contributions to the complex modulus G* [23]
Experimental
SBR preparation
Six different types of star-shaped SSBRs were prepared following the procedure as described below (Table 1) [24]. An autoclave of 10 L as the internal volume with a stirrer was used as a reactor. 777 g of butadiene, 273 g of styrene, 4800 g of cyclohexane, and 0.85 g of 2,2-bis(2-oxolanyl) propane as a polar substance to control the vinyl content were charged in the reactor. The temperature inside the reactor was kept constant at 40°C for 1 min to stabilize the temperature. Each initiator was fed to the reactor to start the polymerization. Due to the heat generation caused by the polymerization, the temperature inside the reactor started to rise, and the final temperature inside the reactor reached around 80 °C. Immediately after the temperature reached a peak, the coupling agent was added to the reactor to carry out the functionalization reaction or termination for 5 min. After 2.1 g of an antioxidant (2,6-di-t-butyl-p-cresol) was added to the polymer solution, the solvent was removed by steam stripping.
The styrene content and vinyl content in butadiene were measured by FT-IR (Fourier-transform infrared spectroscopy) following ISO 21561-2. Mooney viscosities of SBRs were tested by using a Mooney viscometer (Shimadzu, SMV-301) at 100 °C with a large rotor according to ASTM D1646. The value is represented as ML1+4 100 °C. The weight average molecular weight (Mw) of SBR was measured by Gel Permeation Chromatography. 10 mg of each SBR was dissolved in 20 mL of THF (tetrahydrofuran). The solution was filtered with Millex SLFH05010 produced by Merck. The condition of the GPC measurement is depicted in Table 2.
Compound preparation
The compound formulation used for this study is shown in Table 3.
The compounds were mixed in 2 steps following the mixing procedure shown in Table 4. A laboratory-scale internal mixer (Brabender Plasticorder) was used with a 390 mL chamber volume. The fill factor of the internal mixer was fixed to 65%. The temperature of the temperature control unit was set at 100 °C to keep the chamber temperature around 100 °C. The revolution was kept constant at 50 rpm until the time 3:10. After adding the second half of silica and the second half of silane at 3:10, the revolution was varied to reach the target temperature (140, 150, 160 °C) in 1 min. After that, the temperature was kept constant at each target temperature by changing the revolution for 2.5 min to finalize the silanization reaction. After discharging from the mixer, the compounds were sheeted out immediately on a laboratory-scale two-roll mill (Servitech, Polymix 150L) to cool down the compounds and prevent further reactions. The curatives (sulfur, DPG, and CBS) were added on the two-roll mill. The temperature of the rolls was kept at 50 °C.
Sample characterizations
The Payne effect is the reduction of the storage modulus of the filled rubber compound under an increasing strain amplitude. The reduction takes place due to the breakdown of the filler network. Thus, the Payne effect is an indicator of filler–filler interactions [10]. The storage modulus (G′) of the finally mixed uncured and cured rubber compounds was evaluated by using a Rubber Process Analyzer (RPA; Alpha technologies). The storage modulus of the uncured rubber was measured at a temperature of 100 °C, a frequency of 0.5 Hz, and varying strains in the range of 0.56–100%. The storage modulus of the cured rubber was measured at a temperature of 100 °C, a frequency of 0.5 Hz, and varying strains in the range of 0.56–100% after curing the rubber compounds at 160 °C. The Payne effects were calculated from the difference in storage modulus at low strain (0.56%) and high strain (100%).
For silica-filled compounds, it is described in the literature [9] that flocculation can take place during the storage of the vulcanized compounds or during the vulcanization due to the polarity difference between the silica and the polymers. The flocculation influences the physical properties of the silica-filled compound. According to Mihara et al., the flocculation ratio can be measured by RPA monitoring the storage modulus at low strain under isothermal conditions. The flocculation of the compound is measured by using a Rubber Process Analyzer (RPA 2000 of Alpha Technologies) at 100 °C, at a strain of 0.56%, and a test time of 13 min including a pre-heating time of 2 min. The filler flocculation rate (FFR) is calculated from the results fitting with Eqs. 1 and 2 [25].
FFR is the dimensionless flocculation rate, G′0.56(t) is the storage modulus at 0.56% strain at test time t, G′0.56i is the initial storage modulus at ti, and ti is 1 min after pre-heating (Fig. 4).
The vulcanization was performed using a Wickert laboratory press (WLP1600) at 160 °C. The cure curve of each compound was measured at 160 °C, a frequency of 0.83 Hz, and at 2.79% strain by Rubber Process Analyzer (RPA; Alpha technologies). T90 + 5 min is used as the vulcanization time. Viscoelastic properties are measured by Rheometer (ARES-G2: TA Instruments) with a temperature dependence analysis in torsion mode at a strain of 1% and a frequency of 10 Hz. Tan delta (tanδ) at 60 °C was taken as an indicator for the rolling resistance.
Results and discussion
Polymer properties
The microstructure, molecular weight (Mw) detected via GPC, and Mooney viscosity (ML1 + 4) of the six above-introduced SSBRs are depicted in Table 5. The Mooney viscosity and the microstructure were almost constant in each SBR. The molecular weight of those SSBRs which contain an additional functional group (BF4, BF6, BF8) is detected via GPC slightly lower than the corresponding SBR with the same number of arms (F4, F6, and F8), respectively. This is due to the possible interactions between the amine groups in BF-SBRs (BF4, BF6, BF8) and the packing in the column of GPC. It is known from the literature that there is an interaction between the lone pair of the amine and the column packing (polystyrene) [26]. As a result, the evaluated molecular weight of the BF-SBRs seemed to be lower. Given the fact that all SBRs were polymerized at the same conditions, the molecular weight should be the same for the F-SBRs than for the BF-SBRs, and the observed difference comes from the influence of the functional groups.
In addition to the influence of the functional groups, the molecular weight by GPC is influenced by the number of arms as well. Normally, the polymer which has a higher molecular weight creates a larger coil form which gives a shorter retention time in the column indicating a higher molecular weight. However, the size of the polymer of the branched structure does not increase proportionately compared to the linear polymer. For instance, the molecular weight of F8 should be doubled compared to that of F4 because its number of arms is double. However, the measured molecular weight of F8 is just 1.6 times higher than that of F4. Considering that theoretically the functional group E can react with 8 polymer chains, it can be assumed that the polymer was synthesized as expected.
Compound properties
The Payne effect, the polymer–filler interactions, and the flocculation rate of the uncured compounds were investigated to understand the influence of the functional groups. Firstly, the compounds with 6 different SBRs mixed at 160 °C were compared to investigate the influence of the functional groups and branched structure on the polymer–filler interactions and filler–filler interactions. Secondly, all compounds mixed at different temperatures (140, 150, and 160 °C) were used to investigate the effect of the mixing temperature on the polymer–filler interactions.
The concentration and the number of functional groups
It was reported that an amine group can enhance the silanization reaction [20]. Therefore, the concentration of the amine groups in the compound and its influence on different reactions which might take place in the compound should be considered. Therefore, the concentration of the amine groups in the SBR is calculated by Eq. 3 and depicted in Table 6. The concentration of the amine groups in SBR is constant in all BF-SBRs. Therefore, the acceleration of the silanization by the amine groups is expected to be constant in all BF-SBR compounds. The concentration of the alkoxy silyl groups is also shown in Table 6 as a reference.
Influence of the functionalization and number of arms
The strain-dependent storage and loss modulus of the compounds mixed up to 160 °C are depicted in Fig. 5. The dots of blue and green colors represent the results of the compounds with SBR with the functional group only in the center (F4, F6, and F8). The red and yellow colors represent the result of the compounds SBR with functional groups in the center as well as at the chain ends (BF4, BF6, and BF8).
Influence of the functionalization
The storage modulus at a low strain of BF-SBR compounds is always higher than that of the one-end functionalized SBR (F-SBR) compounds. It is expected that BF-SBR compounds showed lower filler–filler interactions because of the presence of the amine groups. Therefore, this result is not aligned with the expectation. There is also a significant difference in the loss modulus (G″). The loss modulus of BF-SBR compounds shows a peak at around 10% strain, whereas no clear peak is observed in the compounds of F-SBR. The loss modulus is related to the breakdown and reformation of the filler network structure [27]. The visible clear peak of G″ in the BF-SBR compounds implies that there is a stronger silica network in the BF-SBR compound compared to the F-SBR compounds.
The storage modulus at 100% is the indicator of the degree of polymer–filler interactions. The storage modulus at 100% of all BF-SBR compounds is significantly higher than that of the F-SBR compounds. Thus, this result indicates that the amine functional groups at the end chain in BF-SBR can increase the polymer–filler interactions in the compound because the difference between F-SBR and BF-SBR is only the presence or absence of the amine groups at each chain end. Furthermore, it is reported that the amine groups start an interaction with silica at 70 °C which is much lower than the mixing temperature of the compound in this study, 160 °C [28]. Therefore, the presence of the amine groups in BF-SBR increases the polymer–filler interactions in the compounds compared to the corresponding F-SBR compounds. Furthermore, all of the BF-SBR compounds show a higher Payne effect than that of the corresponding F-SBR compounds. Given the fact that the result of G″ also indicates a stronger silica network, it can be concluded that the filler–filler interactions of BF-SBR are higher than that of F-SBR even though the polymer–filler interactions are higher in the BF-SBR compounds.
Influence of the number of arms
For the compounds of the F-SBRs, the number of arms does not have any significant impact on G’ at 100% strain (Fig. 6a). It was reported that the branched SBR compounds showed the higher polymer–filler interactions compared to those of the linear type SBR compound [20]. This result led to the assumption that a higher branched SBR should lead to even higher polymer–filler interactions. However, the observed results do not match this assumption.
The storage modulus at 100% is influenced by several factors in the case of the functionalized SBRs with branched structure. The possible factors which influence G’ at 100% strain are depicted in Table 7. The reaction between the alkoxy silyl groups and silanized silica via sulfur derived from TESPT (silica–silane–polymer coupling) is affected by the mixing temperature. It is assumed to be constant in all compounds because the mixing temperature was kept constant for all compounds.
The amount of entanglements is assumed to increase as the number of arms increases. This influences the strain at 100% positively. An interaction between the amine groups and silica can increase the polymer–filler interaction. This interaction can be influenced by the number of the amine groups in one polymer molecule. The interactions or reactions of the alkoxy silyl groups with silica (silica–silane–alkoxy silyl coupling) affect the polymer–filler interactions. When the number of arms in SBR increases, the number of polymer chains which are bound to the alkoxy silyl groups in the center of the polymer increases as well. It was investigated that the reaction rate of the alkoxy silane is influenced by the size of the molecule of the silane [6, 29]. As the alkoxy silyl groups are surrounded by more polymer chains, the accessibility of the alkoxy silyl groups is reduced. Therefore, it is assumed that interactions or reactions of the alkoxy silyl groups and silica decrease with the SBR with a higher number of arms. The last factor, the silanization, is influenced by the presence of the amine groups. This interaction is higher for BF-SBR compounds compared to that of F-SBR. It is proposed that the higher number of amine groups in the branched structure enhances the silanization [20]. However, the concentration of the amine groups is constant in all BF-SBRs in this study. Therefore, the acceleration of the silanization should be constant in all BF-SBR compounds as well.
Considering those assumptions, the contribution of each factor to the storage modulus at 100% strain when the number of arms increases is shown in Table 8. For F-SBRs, the amount of entanglements can increase as the number of arms increases. The interactions between the functional groups in the center of the polymer and silica might be lower in the compound with SBR with a higher number of arms. The combination of both effects results in similar polymer–filler interactions in all F-SBR compounds. Therefore, the storage modulus at 100% strain of F-SBR compounds is more or less constant.
In contrast to this, the storage modulus at 100% strain slightly increases as the number of arms increases in the BF-SBR compounds. Focusing on each factor, the amount of entanglements and the interactions or reactions of the alkoxy silyl groups and silica should be the same as for F-SBRs. The acceleration of the silanization should be the same in all BF-SBRs. Therefore, it can be concluded that the interactions of the amine groups with silica increase the polymer–filler interactions inside a BF-SBR compound as the number of arms becomes higher.
The Payne effect (the difference of storage modulus at 0.56% and 100%) and the storage modulus at 100% strain are presented in Fig. 6. The Payne effect is an indicator of the filler–filler interactions [30]. Focusing on the influence of the number of arms, the difference between each compound is within the variation of the measurement (± 5%). However, there is a tendency for the value of the Payne effect to increase as the number of arms increases for both polymer types, F-SBRs as well as BF-SBRs. Usually, the compound with higher polymer–filler interactions should show lower filler–filler interactions. Therefore, this tendency does not correlate with that of the storage modulus at 100% strain which was constant in all F-SBR compounds. This inconsistency indicates that the impact of each factor on the storage modulus at 100% strain is not the same as the contribution to the filler–filler interactions.
An assumed contribution of each factor to the Payne effect is shown in Table 9. As for F-SBRs, the low degree of interactions or reactions between the alkoxy silyl groups and silica is the only factor which increases the filler–filler interactions. Therefore, it is assumed that its impact on the filler–filler interaction is more significant compared to the impact of other interactions, such as the interactions between the silanol groups of the silica and the amine groups. The interactions of the alkoxy silyl groups with silica can create covalent bonds which are stronger than the hydrogen bonds which amine groups. Therefore, higher filler–filler interactions are observed in the compound with F-SBR with a higher number of arms.
As for BF-SBRs, the higher interactions of the amine groups with the silica are observed because the amount of amine groups increases with increasing number of arms. These higher polymer–filler interactions are supposed to decrease the filler–filler interactions. However, the result is opposite, the Payne effect increases with the higher number of arms in BF-SBRs. These results indicate another reason for the higher filler–filler interactions in the BF-SBR compounds.
As discussed before, there are many reactions and interactions involved in the compound with BF-SBRs. The possible reactions in the compound are depicted in Fig. 7. The amine groups can start their interactions with silica at around 70 °C as reported in the past [28]. The amine groups situated at each arm of BF-SBR can be bound to silica. Then, the alkoxy silyl groups in BF-SBR react or interact with silica. The silane reacts to silica as well more easily when the mixing temperature increases. At a mixing temperature, around 150 or 160 °C, the coupling between polymer and silane via sulfur can take place. Due to this variety of interactions and reactions, the polymer chain can create a network structure during the mixing.
It is proposed that the polymer mobility is enhanced at higher temperatures. The polymer penetrates more easily into the voids of the large clusters. Consequently, clusters are separated by polymer chains resulting in a better micro-dispersion (Fig. 8) [31, 32]. However, in the case of BF-SBR, the mobility of the polymer can be reduced due to the different types of interactions and reactions in the compound. In addition, as the number of arms increases, more polymer–filler interactions caused by the amine groups can take place. It is assumed that a better filler dispersion is prevented by the less mobility of the polymer caused by too much polymer–filler interactions. Furthermore, the network structure is created in polymer which can also prevent silica dispersion. Therefore, the Payne effect of BF-SBR is higher than that of F-SBR, and it is higher as the number of arms increases.
Influence of mixing temperature
The Payne effect and the storage modulus at 100% strain as a function of the compound temperature are presented in Fig. 9. As shown in Table 4, the mixing temperature of the mixer chamber was changed between 140, 150, and 160 °C. At the same time, the real compound temperature was measured by a thermometer after the compound was discharged from the mixer. This real compound temperature is shown in Fig. 9.
The Payne effect of all compounds decreases when the compound temperature is increased from 140 to 150 °C. However, the Payne effect of the compound with the mixing temperature of 160 °C is not further decreased compared to that at 150 °C. This tendency indicates that the mixing temperature of 150 °C is high enough for this compound in order to achieve the higher degree of silanization which results in the lower filler–filler interactions [33]. The storage modulus at 100%, the indicator of polymer–filler interactions, becomes higher as the compound temperature increases. The silanization and the coupling reaction between silane and polymer are accelerated when the mixing temperature increases. The reactions or interactions of polymer and silica via the functional groups in SBR can also be accelerated as the mixing temperature increases. Those can be the reasons why the G’ at 100% is increased with higher mixing temperature.
The difference between F-SBR and BF-SBR compounds with regard to the resulting Payne effects becomes significant at the highest investigated mixing temperature. For the compound mixed at 140 °C, the Payne effect of all compounds is almost the same. However, the BF-SBR compounds show higher Payne effects compared to the F-SBR compounds in the case of investigated mixing temperatures at 150 and 160 °C. This tendency matches the theory discussed above. As the temperature increases, more reactions are involved, e.g., the silanization, the coupling between polymer and silane, and the interactions or reactions between the alkoxy silyl groups and silica. For BF-SBR compounds, the mobility of the polymer chain is reduced due to the higher polymer–filler interactions caused by the functional groups. These interactions increase further at higher mixing temperature. Therefore, a good silica dispersion cannot be achieved for the compound mixed at higher temperature. Therefore, BF-SBR compounds mixed at higher temperature show higher filler–filler interactions compared to that of F-SBRs.
Filler flocculation rate
The result of the flocculation is shown in Fig. 10. The filler flocculation rate value is plotted as a function of the investigated mixing temperature (Fig. 11). The filler flocculation rate of all compounds becomes lower as the mixing temperature increases. It is reported that a higher degree of silanization and a higher polymer–filler interaction lead to a reduced tendency for flocculation [34]. The silanization and the sulfur coupling between polymer and silanized silica is accelerated with higher temperature. The higher polymer–filler interactions caused by the silanization and the higher sulfur coupling prevent the silica to re-agglomerate. Therefore, the filler flocculation rate of all compounds is reduced with the higher mixing temperature.
The effect of the amine functionalization on the flocculation can be discussed by comparing F-SBR compounds with their corresponding BF-SBR compounds. At a mixing temperature of 140 °C, the filler flocculation rate is lower in the BF-SBR compounds compared to their corresponding F-SBR compounds. This difference in the filler flocculation rate values between BF-SBR and F-SBR compounds becomes smaller as the investigated mixing temperature increases.
In order to discuss which factor influences the polymer–filler interactions at each temperature, the impact of each factor is depicted in Table 10.
It was found in the literature that the optimum silanization temperature is around 150–160 °C [35]. Considering this temperature range, there might be some unreacted silane in the compound mixed only at 140 °C. As the amine groups start interacting with silica at around 70 °C, it can be expected that these interactions between the amine groups and silica also take place in the compound mixed at 140 °C. Therefore, the impact of the amine and silica interactions on the polymer–filler interactions is considered to be large in the compound mixed at 140 °C. Therefore, the BF-SBR compounds mixed at 140 °C show a lower filler flocculation rate compared to that of F-SBR.
As the mixing temperature increases, the degree of silanization and the interaction between the alkoxy silyl groups and silica become higher. Furthermore, an additional polymer–filler coupling via sulfur derived from TESPT starts in the compound. These interactions result in covalent bonds between polymer and silica, whereas the amine groups can create only hydrogen bonds. These hydrogen bonds are reversible interactions and, therefore, weaker than the covalent bonds. As a result of this, in the compounds mixed at 150 and 160 °C, the polymer–filler interactions caused by TESPT and the alkoxy silyl groups become the major factors increasing the polymer–filler interactions. Consequently, there is no significant difference between F-SBR and BF-SBR compounds.
Evaluating the SBRs with different number of arms, no significant difference is observed in filler flocculation rate. Particularly for BF-SBRs, it is expected that the SBR with a higher number of arms should show a lower filler flocculation rate. Hence, there are higher polymer–filler interactions caused by the higher amount of amine groups in the branched SBR with a higher number of arms. However, the observed result for the filler flocculation rate is not consistent with this assumption. It is assumed that the flocculation is also influenced by the degree of dispersion in the compound. In the compound with the worse silica dispersion, the silica might form even larger clusters more easily. The Payne effect of the BF-SBR compounds is higher for the SBR with a higher number of arms. Therefore, the combination of the higher polymer–filler interactions and the higher filler–filler interactions results in a more or less constant filler flocculation rate in the BF-SBR compounds.
Cure curve
The cure curve of the compounds mixed at 160 °C is depicted in Fig. 12a. The torque indicates the crosslinking density caused by polymer–polymer and silica–silane–polymer coupling but also contains a filler–filler network contribution [36]. A lot of interactions or reactions cause the higher torque for the compound with a functionalized SBR, such as interactions of functional groups and silica, interactions between functional groups and silica or silanized silica. The flocculation which takes place at the beginning of the curing can also contribute to the torque. The maximum torque of the BF-SBR compounds is slightly higher than that of the corresponding F-SBR. Considering the fact that there is no significant difference between the filler flocculation rate of the BF-SBR compound and that of the F-SBR compound mixed at 160 °C, the flocculation cannot be the main reason for this difference in the cure curve. As shown in Fig. 9, the storage modulus at 100% strain is always higher in the BF-SBR compounds compared to the F-SBR compounds. These higher polymer–filler interactions in BF-SBR increase the cure torque.
The minimum torque of each compound is depicted in Fig. 12c. As the torque quickly rises at the beginning of the cure curve of these compounds (Fig. 12b), the ML values do not show such a tendency. The difference between the maximum torque (MH) and the minimum torque (ML) is plotted as a function of the mixing temperature in Fig. 12d. The torque of the BF-SBR compound is always slightly higher than that of the F-SBR compound. Considering that the storage modulus at 100% strain of the BF-SBR compounds is higher compared to those of F-SBR compounds, the polymer–filler interactions contribute to the higher MH-ML in the BF-SBR compounds compared to the F-SBR compounds.
Payne effect of the cured compounds
The storage modulus of the compounds mixed at 160 °C as a function of the strain is depicted in Fig. 13. The storage modulus of the F-SBR as well as the BF-SBR compounds is slightly higher as the number of the arms in SBR increases. The higher storage modulus is observed in the BF-SBR compounds compared to their corresponding F-SBR compounds which corresponds to the results of the uncured compounds (Fig. 9).
The Payne effect as the function of the mixing temperature is summarized in Fig. 14a. All cured compounds mixed at higher temperature show a lower Payne effect. In the uncured compounds, the Payne effect only significantly decreases as the mixing temperature increases from 140 to 150 °C, but there was no significant difference between the results at 150 °C and 160 °C (Fig. 9). The decency of the Payne effect of the cured compound does not fully match the results of the uncured compound. It has to be considered that the Payne effect of the cured compound is also influenced by the filler flocculation rate because the flocculation takes place during the curing or the storage. Flocculation also can take place before the Payne effect measurement of the uncured compounds. However, the Payne effect of the uncured compound was measured right after the mixing; therefore, the influence of the flocculation on the Payne effect of the uncured compound can be neglected. As depicted in Fig. 11, the filler flocculation rate decreases with the higher mixing temperature. This result indicates that the flocculation is reduced in the compound mixed at higher temperature due the higher polymer–filler interactions. Therefore, the Payne effect of the cured compounds decreases as the mixing temperature increases from 150 to 160 °C which is slightly different from the tendency observed in the uncured compounds.
Focusing on the number of arms, the highest Payne effect is observed in the compound of the BF-SBR series for the SBR with 8 arms (BF8) and in the F-SBR series also for the compound with 8 arms (F8). This tendency is similar to that of the uncured compound.
The influence of the mixing temperature on the storage modulus at 100% is shown in Fig. 14b. Considering that ± 5% is the measurement error, there is no significant difference between all compounds. However, there is a trend that G’ increases as the mixing temperature rises. This tendency matches the results of the uncured compounds. This is due to the higher polymer–filler interactions caused by a silica–silane–polymer coupling or a silica–silane–functional group coupling. Both coupling reactions are enhanced at higher mixing temperature. Therefore, a higher storage modulus is observed in the compounds mixed at higher temperature.
As discussed before, it can be assumed that the storage modulus at 100% strain should be influenced by the amount of amine–filler interactions. In that case, the storage modulus of BF-SBR compounds should be higher than that of F-SBR compounds. The compound of BF-SBR with a higher number of arms should also show the higher storage modulus, because of the higher amount of amine in SBR. However, there is neither a significant difference in G’ of BF-SBR and F-SBR nor in G’ of BF-SBRs with different number of arms. As discussed before, the interactions between the amine groups and silica are relatively weak compared to the other reactions which can create covalent bonds. The amine–filler interactions can be embedded in a contribution of other reactions and the polymer–polymer network created during vulcanization.
Tensile strength
The tensile properties of each compound are depicted in Table 11. Since the elongation did not reach 300%, the modulus at 200% strain (M200) is used instead of M300. M200 of the compound as the function of the investigated mixing temperature is depicted in Fig. 15. The blue and green color bars represent the data of F-SBR compounds, and those of pink and yellow color represent the data of BF-SBR compounds.
The tensile strength of all BF-SBR compounds is higher than that of F-SBR compounds mixed at 150 °C and 160 °C. This is because of the higher filler–polymer interactions in the compound of BF-SBR than that of the corresponding F-SBR compounds which was observed in the storage modulus of the uncured samples. Although the influence of the amine groups on the polymer–filler interactions is not visible in the Payne effect results of the cured compound, it affects the tensile properties. The compounds of BF-SBR with a higher number of arms show a higher value of tensile strength. This also corresponds to the formation of polymer–filler interactions in the uncured compounds. The higher polymer–filler interactions are indicated by the highest G′ at 100% strain for BF-SBR with the highest number of arms. These increased polymer–filler interactions are not visible in the cured compound; however, they lead to the higher tensile strength.
The elongation at break is summarized in Fig. 16. Considering the error bar range, the difference of each SBR compound is not significant. However, all SBR compounds show the highest elongation with the mixing temperature of 140 °C and the lowest elongation with the mixing temperature of 160 °C. This is due to the higher polymer–filler interactions with the highest mixing temperature which was already discussed before.
Rolling resistance indicator
The tanδ at high temperature (50–70 °C) is used as an indicator of the rolling resistance. The tanδ can be reduced by better silica dispersion and decreasing mobility of the polymer chain end [6, 29]. The functionalized SBR is expected to improve the silica dispersion and to reduce the mobility of polymer chains by increasing polymer–filler interactions. Tanδ at 60 °C as a function of the mixing temperature is depicted in Fig. 17. Focusing on the influence of the mixing temperature, tanδ of all compounds decreases as the mixing temperature increases. The higher storage modulus at 100% strain is observed in the cured compound with higher mixing temperature due to the higher polymer–filler interactions. This results in the lowest Payne effect (Fig. 14). Therefore, the compound mixed at high temperature shows the lowest tanδ.
The tanδ of the BF-SBR compounds is the same or slightly lower compared to that of the F-SBR compounds. As discussed before, the Payne effect and the polymer–filler interactions influence tanδ. Therefore, the compound with the lowest Payne effect value should show the lowest tanδ. However, the Payne effect of the BF-SBR compounds is higher than that of the F-SBR compounds (Fig. 14) which is not consistent with the result where the tanδ of BF-SBR is lower. At the same time, the polymer–filler interactions of BF-SBR in the uncured compound are higher than that of the F-SBR compound which matches the tendency of tanδ.
In order to understand this, the possible polymer interactions in the BF-SBR compounds compared to the F-SBR are summarized in Table 12. The indicator of polymer–filler interactions (G′ at 100% strain) and the Payne effect are also depicted in the same table.
As discussed before, the polymer–filler interactions in BF-SBR are considered to be higher than those in the F-SBR compound due to the presence of the amine groups. However, the influence of these interactions is only visible in the uncured compound but not visible in the cured compound. This might be explained by the assumption that the interactions between the amine groups and silica are relatively weak, and that they cannot be separated from the other dominant reactions. Assuming that the polymer–filler interactions are higher in the cured compound with BF-SBR, these interactions are the cause of the lower tanδ of BF-SBR compounds.
The number of arms does not influence tanδ, considering the measurement error of ± 5%. It was reported that the higher amount of amine groups in the branched SBRs decreases the tanδ compared to that of the linear type SBR [20]. However, the higher number of the amine groups does not decrease tanδ in this study. As discussed, the higher amount of the amine groups can prevent the silica dispersion during the mixing and results in the higher Payne effect (Fig. 14). At the same time, there should be higher polymer–filler interactions in the BF-SBR compound with a higher number of arms. The combination of these effects results in a more or less constant value of tanδ.
Conclusion
The influence of the number of end-chain amine-functionalized arms of SSBR was investigated in this study. The higher number of amine groups in SBR with higher number of arms increased the storage modulus at 100% strain, indicating higher polymer–filler interactions. However, these high interactions did not decrease the Payne effect, an indication for the filler–filler interactions and with this, for the micro-dispersion of the filler. The high polymer–filler interactions due to the high amount of amine groups in the SBR with high number of arms prevent a good silica micro-dispersion during the mixing. At the same time, the effect of the alkoxy silyl group on the polymer–filler interactions decreases. This is because more polymer chains surround the alkoxy silyl group and prevent the interactions between alkoxy silyl groups and silica. The higher number of amine groups decreases also the flocculation rate in the compounds. These combinations of the higher polymer–filler interactions, the higher Payne effect, and the lower flocculation rate resulted in the more or less same value of tanδ when the number of arms increases.
References
Transportation Research Board (2006) Tires and passenger vehicle fuel economy. https://nap.nationalacademies.org/
Roland R (February 12, 1992) EP Patent 0501227 (to MICHELIN and CIE)
Reuvekamp LAEM, ten Brinke JW, Van Swaaij PJ, Noordermeer JWM (2002) Effects of mixing conditions reaction of TESPT silane coupling agent during mixing with silica filler and tire rubber. KGK-Kautschuk und Gummi Kunststoffe 55:41–47
Kaewsakul W, Sahakaro K, Dierkes WK, Noordermeer JWM (2012) Optimization of mixing conditions for silica-reinforced natural rubber tire tread compounds. Rubber Chem Technol 85:277–294. https://doi.org/10.5254/rct.12.88935
Blume A (2011) Kinetics of the silica-silane reaction. KGK, Kaut Gummi Kunstst 64:38–43
El-Roz M, Blume A, Thibault-Starzyk F (2014) Infrared study of the silica/silane reaction. KGK, Kaut Gummi Kunstst 67:53–57
ten Brinke JW, Debnath SC, Reuvekamp LAEM, Noordermeer JWM (2003) Mechanistic aspects of the role of coupling agents in silica-rubber composites. Compos Sci Technol 63:1165–1174. https://doi.org/10.1016/s0266-3538(03)00077-0
ten Brinke JW, Van Swaaij PJ, Reuvekamp LAEM, Noordermeer JWM (2003) The influence of silane sulfur and carbon rank on processing of a silica reinforced tire tread compound. Rubber Chem Technol 76:12–35. https://doi.org/10.5254/1.3547728
Mihara S (2009) Reactive processing of silica-reinforced tire rubber: new insight into the time- and temperature-dependence of silica rubber interaction. University of Twente, Enschede. https://doi.org/10.3990/1.9789036528399
Hayichelaeh C, Reuvekamp LAEM, Dierkes WK, Blume A, Noordermeer JWM, Sahakaro K (2017) Reinforcement of natural rubber by silica/silane in dependence of different amine types. Rubber Chem Technol 90:651–666. https://doi.org/10.5254/rct.82.83708
Baczek SK, Anderson JN, Adams HE (1976) Functionality distribution of hydroxyl-terminated polybutadienes by gel permeation chromatography. II. Measurements for commercial polymers. Rubber Chem Technol 49:1250–1258. https://doi.org/10.5254/1.3535012
Sierra CA, GaláN C, GóMez Fatou JM, Quiteria VRS (1995) Dynamic-mechanical properties of tin-coupled SBRs. Rubber Chem Technol 68:259–266. https://doi.org/10.5254/1.3538740
Zhang S, Zhao S, Zhang X, Zhang L, Wu Y (2014) Preparation, structure, and properties of end-functionalized miktoarms star-shaped polybutadiene-sn-poly(styrene-butadiene) rubber. J Appl Polym Sci 131:40002. https://doi.org/10.1002/app.40002
Tsutsumi F, Sakakibara M, Oshima N (1990) Structure and dynamic properties of solution SBR coupled with tin compounds. Rubber Chem Technol 63:8–22. https://doi.org/10.5254/1.3538245
Sone T, Yuasa T (2010) Study on solution SBR with modified chain end for fuel efficient Tire. Nippon Gomu Kyokaishi 83:103–108. https://doi.org/10.2324/gomu.83.103
Döring C, Thiele SKH, Heidenreich D (2015) Multi-functionalized SSBR and compound vulcanizate performance characteristics. Rubber World 251:35–41
Hayashi M, Hama H, Inagaki K (2011) Development and foresight of solution SBR for energy-saving tires. Sumitomokagaku I:31–38
Seo B, Kim K, Lee H, Lee JY, Kwag GH, Kim W (2015) Effect of styrene-butadiene rubber with different macrostructures and functional groups on the dispersion of silica in the compounds. Macromol Res 23:466–473. https://doi.org/10.1007/s13233-015-3055-8
Zhao S, Zhang X, Jin G (2003) Influence of molecular structure of star S-SBR on its properties. J Appl Polym Sci 89:2311–2315. https://doi.org/10.1002/app.11928
Yamada C, Yasumoto A, Matsushita T, Blume A (2022) Influence of functionalized S-SBR on silica–filled rubber compound properties. Funct Compos Mater 3:6. https://doi.org/10.1186/s42252-022-00034-8
Payne AR, Whittaker RE (1971) Low strain dynamic properties of filled rubbers. Rubber Chem Technol 44:440–478
Medalia AI (1973) Elastic modulus of vulcanizates as related to carbon black structure. Rubber Chem Technol 46:877–896
Luginsland HD, Fröhlich J, Wehmeier A (2002) Influence of different silanes on the reinforcement of silica-filled rubber compounds. Rubber Chem Technol 75:563–579
Ishizaka T, Yamazaki H, Yamada C (March 13, 2013) JP Patent 6924003 (to Asahi Kasei Corporation)
Jin J (2020) Influence of compounding and mixing on filler dispersion and curing behavior of silica compounds. PhD Thesis, University of Twente, The Netherland https://doi.org/10.3990/1.9789036549264
Oguri N, Onishi A, Nakahashi K, Uchino S (1993) Separation of polar oligomers by size exclusion chromatography. Bunseki Kagaku 42:T37–T41. https://doi.org/10.2116/bunsekikagaku.42.2_T37
Lin CJ, Hergenrother WL, Alexanian E, BöHm GGA (2002) On the filler flocculation in silica-filled rubbers part I. Quantifying and tracking the filler flocculation and polymer-filler interactions in the unvulcanized rubber compounds. Rubber Chem Technol 75:865–890. https://doi.org/10.5254/1.3547689
Chigusa Y (2023) Influence of functionalized S–SBR on silica–filled rubber compound properties. PhD thesis, University of Twente, The Netherland, https://doi.org/10.3990/1.9789036556422
Akutagawa K (2017) Technology for reducing tire rolling resistance. Tribol Online 12:99–102. https://doi.org/10.2474/trol.12.99
Payne AR (1962) The dynamic properties of carbon black-loaded natural rubber vulcanizates. Part I. J Appl Polym Sci 6:57–63. https://doi.org/10.1002/app.1962.070061906
Jin J, Kaewsakul W, Noordermeer JWM, Dierkes WK, Blume A (2021) Macro- and micro-dispersion of silica in tire tread compounds: Are they related? Rubber Chem Technol 94:355–375. https://doi.org/10.5254/rct.20.80365
Grunert F (2018) Analytical method development to predict the in-rubber dispersibility of silica. PhD Thesis, University of Twente, The Netherlands https://doi.org/10.3990/1.9789036546553
Reuvekamp LAEM (2003) Reactive mixing of silica and rubber for tyres and engine mounts: influence of dispersion morphology on dynamic mechanical properties. PhD Thesis, University of Twente, The Netherlands. https://research.utwente.nl/files/255318645/thesis_L_Reuvekamp.pdf
Jin J, Noordermeer JWM, Dierkes WK, Blume A (2019) The origin of marching modulus of silica-filled tire tread compounds. Rubber Chem Technol 93:378–394. https://doi.org/10.5254/rct.19.80453
Reuvekamp LAEM, ten Brinke JW, Van Swaaij PJ, Noordermeer JWM (2002) Effects of time and temperature on the reaction of tespt silane coupling agent during mixing with silica filler and tire rubber. Rubber Chem Technol 75:187–198. https://doi.org/10.5254/1.3544972
Bisschop R, Grunert F, Ilisch S, Stratton T, Blume A (2021) Influence of molecular properties of SSBR and BR types on composite performance. Polym Testing 99:107219. https://doi.org/10.1016/j.polymertesting.2021.107219
Acknowledgements
The authors gratefully acknowledge financial and materials support from Asahi Kasei Corporation (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamada, C., Yasumoto, A. & Blume, A. Influence of the number of end-chain amine-functionalized arms of star-shaped functionalized SBR. Polym. Bull. 81, 11049–11075 (2024). https://doi.org/10.1007/s00289-024-05160-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05160-w