Abstract
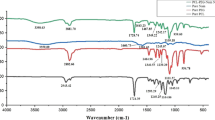
This study aimed to investigate the antibacterial properties of polyvinyl alcohol/chitosan/potassium permanganate composite nanofibers in different concentrations against gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa. For this purpose, the nanofibers above were first made using the electrospun method. Then, the antibacterial effects of the nanofibers were evaluated by antibiogram test. Scanning electron microscopy (SEM) results showed that the diameter of polyvinyl alcohol/chitosan nanofibers ranges from 186.52 to 96.55 nm and has a length of several micrometers. The images of SEM analysis also showed that nanofibers have a smooth surface without any beads. Moreover, the results of X-map analysis have shown that the addition of potassium permanganate slightly decreases the uniform distribution of elements along the length of the nanofibers. Energy-disperse X-ray (EDX) spectroscopy revealed that carbon is the most abundant element in the nanofibers. Fourier-transform infrared spectroscopy (FT-IR) analysis revealed the occurrence of new bonds of elements after the addition of potassium permanganate. The antibacterial test exhibited the most antibacterial properties of nanofibers containing 1 ml of potassium permanganate with a concentration of 1% against both E. coli and P. aeruginosa. It is worth mentioning that the antibacterial property of nanofibers against Escherichia coli was higher than P. aeruginosa. So, using the above-mentioned nanofibers could be a promising antibacterial strategy against infectious bacteria in wounds.







Similar content being viewed by others
Availability of data and materials
I confirm that I have included a data availability statement in my main manuscript file.
References
O’Neill, J (2015) Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist
Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX (2017) Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122:34–47
Zhang G, Shen X, Chen S, Liang L, Luo Y, Yu J, Lu J (2019) DSM: A deep supervised multi-scale network learning for skin cancer segmentation. IEEE Access 7:140936–140945
Yoon J, Jeong B, Lee WH, Kim J (2018) Tracing the evolving trends in electronic skin (e-skin) technology using growth curve and technology position-based patent bibliometrics. IEEE Access 6:26530–26542
Singh VK, Abdel-Nasser M, Rashwan HA, Akram F, Pandey N, Lalande A et al (2019) FCA-net: adversarial learning for skin lesion segmentation based on multi-scale features and factorized channel attention. IEEE Access 7:130552–130565
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193):314–321
Harding KG, Morris HL, Patel GK (2002) Clinical review Healing chronic wounds. Br Med J 324:160–163
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49(1):711–745
Wu P, Grainger DW (2006) Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 27(11):2450–2467
Hetrick EM, Schoenfisch MH (2006) Reducing implant-related infections: active release strategies. Chem Soc Rev 35(9):780–789
Shin YM, Hohman MM, Brenner MP, Rutledge GC (2001) Experimental characterization of electrospinning: the electrically forced jet and instabilities. Polymer 42(25):09955–09967
Smit E, Bűttner U, Sanderson RD (2005) Continuous yarns from electrospun fibers. Polymer 46(8):2419–2423
Li D, Xia Y (2004) Electrospinning of nanofibers: reinventing the wheel? Adv Mater 16(14):1151–1170
Bowler PG, Duerden BI, Armstrong DG (2001) Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 14(2):244–269
Lu X, Wang C, Wei Y (2009) One-dimensional composite nanomaterials: Synthesis by electrospinning and their applications. Small 5(21):2349–2370
Gaharwar AK, Mukundan S, Karaca E, Dolatshahi-Pirouz A, Patel A, Rangarajan K et al (2014) Nanoclay-enriched poly (ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A 20(15–16):2088–2101
Luo CJ, Stoyanov SD, Stride E, Pelan E, Edirisinghe M (2012) Electrospinning versus fibre production methods: from specifics to technological convergence. Chem Soc Rev 41(13):4708–4735
Reneker DH, Yarin AL (2008) Electrospinning jets and polymer nanofibers. Polymer 49(10):2387–2425
Hosseini Ravandi SA, Gandhimathi C, Valizadeh M, Ramakrishna S (2013) Application of electrospun natural biopolymer nanofibers. Curr Nanosci 9(4):423–433
Bernard M, Jubeli E, Pungente MD, Yagoubi N (2018) Biocompatibility of polymer-based biomaterials and medical devices–regulations, in vitro screening and risk-management. Biomater Sci 6(8):2025–2053
Ghobril C, Grinstaff MW (2015) The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem Soc Rev 44(7):1820–1835
Boateng JS, Matthews KH, Stevens HN, Eccleston GM (2008) Wound healing dressings and drug delivery systems: a review. J Pharm Sci 97(8):2892–2923
Jayakumar R, Prabaharan M, Kumar PS, Nair SV, Tamura H (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29(3):322–337
Chattopadhyay S, Raines RT (2014) Collagen-based biomaterials for wound healing. Biopolymers 101(8):821–833
Moura LI, Dias AM, Carvalho E, de Sousa HC (2013) Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater 9(7):7093–7114
Hu MS, Maan ZN, Wu JC, Rennert RC, Hong WX, Lai TS et al (2014) Tissue engineering and regenerative repair in wound healing. Ann Biomed Eng 42(7):1494–1507
Campoccia D, Montanaro L, Arciola CR (2013) A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 34(34):8533–8554
Parani M, Lokhande G, Singh A, Gaharwar AK (2016) Engineered nanomaterials for infection control and healing acute and chronic wounds. ACS Appl Mater Interfaces 8(16):10049–10069
Pirsa S, Mohammadi B (2021) Conducting/biodegradable chitosan-polyaniline film; Antioxidant, color, solubility and water vapor permeability properties. Main Group Chem 20(2):133–147
Luo Y, Wang Q (2013) Recent advances of chitosan and its derivatives for novel applications in food science. J Food Process Beverages 1(1):1–13
Je JY, Kim SK (2006) Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J Agric Food Chem 54(18):6629–6633
Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromol 4(6):1457–1465
Wang T, Zhu XK, Xue XT, Wu DY (2012) Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohyd Polym 88(1):75–83
Takeda M, Kondo K, Yamada M, Koizumi JI, Mashima T, Matsugami A, Katahira M (2010) Solubilization and structural determination of a glycoconjugate which is assembled into the sheath of Leptothrix cholodnii. Int J Biol Macromol 46(2):206–211
Baxter RM, Dai T, Kimball J, Wang E, Hamblin MR, Wiesmann WP et al (2013) Chitosan dressing promotes healing in third degree burns in mice: gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J Biomed Mater Res Part A 101(2):340–348
Kossovich LY, Salkovskiy Y, Kirillova IV (2010) Electrospun chitosan nanofiber materials as burn dressing. In: 6th world congress of biomechanics (WCB 2010). August 1–6, 2010 Singapore. Springer, Berlin, Heidelberg, pp 1212–1214
Goy RC, Morais ST, Assis OB (2016) Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev Bras 26:122–127
Xing Y, Xu Q, Li X, Chen C, Ma L, Li S et al (2016) Chitosan-based coating with antimicrobial agents: preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int J Polym Sci. https://doi.org/10.1155/2016/4851730
Divya K, Vijayan S, George TK, Jisha MS (2017) Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers Polym 18(2):221–230
Mirzaei E, Faridi-Majidi R, Shokrgozar MA, Asghari Paskiabi F (2014) Genipin cross-linked electrospun chitosan-based nanofibrous mat as tissue engineering scaffold. Nanomed J 1(3):137–146
Kang YO, Yoon IS, Lee SY, Kim DD, Lee SJ, Park WH, Hudson SM (2010) Chitosan-coated poly (vinyl alcohol) nanofibers for wound dressings. J Biomed Mater Res Part B: Appl Biomater: Offic J Soc Biomater, Jpn Soc Biomater, Aust Soc Biomater Korean Soc Biomater 92(2):568–576
Hong KH, Park JL, Sul IH, Youk JH, Kang TJ (2006) Preparation of antimicrobial poly (vinyl alcohol) nanofibers containing silver nanoparticles. J Polym Sci, Part B: Polym Phys 44(17):2468–2474
Zhang Y, Huang X, Duan B, Wu L, Li S, Yuan X (2007) Preparation of electrospun chitosan/poly (vinyl alcohol) membranes. Colloid Polym Sci 285(8):855–863
Ding B, Kim HY, Lee SC, Lee DR, Choi KJ (2002) Preparation and characterization of nanoscaled poly (vinyl alcohol) fibers via electrospinning. Fibers Polym 3(2):73–79
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6(1):29–40
Sánchez-Saldaña L, Sáenz-Anduaga E (2015) Antisépticosy desinfectantes
Majtan J (2011) Methylglyoxal—a potential risk factor of Manuka honey in healing of diabetic ulcers. Evid-Based Complement Altern Med 295494.
Anderson I (2003) Should potassium permanganate be used in wound care? Nurs Times 99(31):61–61
Chirapapaisan C, Prabhasawat P, Srivannaboon S, Roongpoovapatr V, Chitsuthipakorn P (2018) Ocular injury due to potassium permanganate granules. Case Rep Ophthalmol 9(1):138–143
Scott KJ, McGlasson WB, Roberts EA (1970) Potassium permanganate as an ethylene absorbent in polyethylene bags to delay ripening of bananas during storage. Aust J Exp Agric 10(43):237–240
Goldstein J (ed) (2012) Practical scanning electron microscopy: electron and ion microprobe analysis. Springer
Pirsa S, Asadi S (2021) Innovative smart and biodegradable packaging for margarine based on a nano composite polylactic acid/lycopene film. Food Add Contam: Part A 38(5):856–869
Meydanju N, Pirsa S, Farzi J (2022) Biodegradable film based on lemon peel powder containing xanthan gum and TiO2–Ag nanoparticles: Investigation of physicochemical and antibacterial properties. Polym Test 106:107445
Yorghanlu RA, Hemmati H, Pirsa S, Makhani A (2022) Production of biodegradable sodium caseinate film containing titanium oxide nanoparticles and grape seed essence and investigation of physicochemical properties. Polym Bull 79(10):8217–8240
Shekh MI, Amirian J, Stadler FJ, Du B, Zhu Y (2020) Oxidized chitosan modified electrospun scaffolds for controllable release of acyclovir. Int J Biol Macromol 151:787–796
Shekh MI, Patel KP, Patel RM (2018) Electrospun ZnO nanoparticles doped core–sheath nanofibers: characterization and antimicrobial properties. J Polym Environ 26:4376–4387
Zhou J, Yao D, Qian Z, Hou S, Li L, Jenkins ATA, Fan Y (2018) Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 161:11–23
Theron A, Zussman E, Yarin AL (2001) Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology 12(3):384
Deitzel JM, Kleinmeyer JD, Hirvonen JK, Tan NB (2001) Controlled deposition of electrospun poly (ethylene oxide) fibers. Polymer 42(19):8163–8170
Dersch R, Liu T, Schaper AK, Greiner A, Wendorff JH (2003) Electrospun nanofibers: Internal structure and intrinsic orientation. J Polym Sci, Part A: Polym Chem 41(4):545–553
Sun Z, Zussman E, Yarin AL, Wendorff JH, Greiner A (2003) Compound core–shell polymer nanofibers by co-electrospinning. Adv Mater 15(22):1929–1932
Dubey P, Bhushan B, Sachdev A, Matai I, Uday Kumar S, Gopinath P (2015) Silver‐nanoparticle‐incorporated composite nanofibers for potential wound‐dressing applications. J Appl Polym Sci 132(35):1–12
Ueno H, Mori T, Fujinaga T (2001) Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev 52(2):105–115
Chen CS, Liau WY, Tsai GJ (1998) Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. J Food Prot 61(9):1124–1128
Fang SW, Li CF, Shih DY (1994) Antifungal activity of chitosan and its preservative effect on low-sugar candied kumquat. J Food Prot 57(2):136–140
Jung BO, Kim CH, Choi KS, Lee YM, Kim JJ (1999) Preparation of amphiphilic chitosan and their antimicrobial activities. J Appl Polym Sci 72(13):1713–1719
Unnithan AR, Barakat NA, Pichiah PT, Gnanasekaran G, Nirmala R, Cha YS et al (2012) Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym 90(4):1786–1793
Zheng LY, Zhu JF, Sun KS (2000) Antimicrobial activity of chitosan. Mater Sci Eng-Hangzhou 18(2):22–24
Zhang S, Li J, Li J, Du N, Li D, Li F, Man J (2020) Application status and technical analysis of chitosan-based medical dressings: a review. RSC Adv 10(56):34308–34322
Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano AP, Hamblin MR (2006) Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 27(22):4157–4164
Vicentini DS, Smania A Jr, Laranjeira MC (2010) Chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Mater Sci Eng, C 30(4):503–508
Tan L, Hu J, Huang H, Han J, Hu H (2015) Study of multi-functional electrospun composite nanofibrous mats for smart wound healing. Int J Biol Macromol 79:469–476
Hu N, Wu XH, Liu R, Yang SH, Huang W, Jiang DM et al (2015) Novel application of vacuum sealing drainage with continuous irrigation of potassium permanganate for managing infective wounds of gas gangrene. J Huazhong Univer Sci Technol [Med Sci] 35(4):563–568
Rai V (2020) What is the evidence for the use of potassium permanganate for wound care? Drug Ther Bull 58(5):71–74
Kegere J, Ouf A, Siam R, Mamdouh W (2019) Fabrication of poly (vinyl alcohol)/chitosan/bidens pilosa composite electrospun nanofibers with enhanced antibacterial activities. ACS Omega 4(5):8778–8785
Chen W, Wu Q, Zhang J, Wu H (2008) Antibacterial mechanism of chitosan. Wei Sheng Wu Xue Bao = Acta Microbiol Sini 48(2):164–168
Freeman F (1973) Postulated intermediates and activated complexes in the permanganate ion oxidation of organic compounds. Rev React Spec Chem React 1(2):179–226
Funding
Funding information is not applicable/no funding was received.
Author information
Authors and Affiliations
Contributions
Mrs. Asadi established the setup of the experimental work. Dr. Hamidinezhad and Dr. Karimian managed the experimental work and wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Consent for publication
The Authors as a result of this consent to the publication of the work in any Journal of Materials Science publications.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asadi, S., Hamidinezhad, H. & Karimian, M. Evaluation of antibacterial properties of polyvinyl alcohol/chitosan/potassium permanganate electrospun nanofibers. Polym. Bull. 81, 2627–2642 (2024). https://doi.org/10.1007/s00289-023-04863-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04863-w