Abstract
Nanocomposites based on acrylonitrile butadiene rubber (NBR) and ethylene–proplene diene monomer (EPDM) (50/50) filled with different concentrations of nano-waste magnetic filler (nWMF) up to 120 phr were prepared by two laboratory two roll mill mixer. nWMF was characterized using XRD, TEM and VSM. The prepared nanocomposites were investigated by different techniques: SEM, TGA, and DSC. In addition the rheological, mechanical, dielectric and magnetic properties were studied. However, the miscibility of NBR/EPDM/nWMF nanocomposites is enhanced after the addition of nano-waste magnetic filler (nWMF). Also, it is found that the thermal stability of NBR/EPDM blend is improved by increasing nWMF loadings. The incorporation of nWMF in rubber matrix increased minimum/maximum torque, cure rate index and reinforcing factor but decreased both optimum cure and scorch time. The mechanical properties showed an enhancement up to 40 phr of nWMF. Further, the magnetization shows a continuous increase with nWMF loading. In addition, the values ε′ and ε″ increased upon rising nWMF concentration. The nanocomposite containing 40 phr shows promising mechanical and dielectric properties which validate its use for insulating and antistatic purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During processing, natural resources used by industrial units produce byproducts which are designated as “waste” as long as no use for this byproduct exists. Besides, there is no way to prevent production of a waste, but it can be used as a resource for some other products.
Steel is a versatile commodity that plays a major part in everyday life for example; food cans, household containers, automobiles and office buildings. Steel represents the largest category of metals in the municipal solid waste (MSW) and industrial waste streams. Steel for ages has been a recycled material throughout the world [1, 2].
In steel industries, there are many sources of solid wastes which can be recognized as; byproduct plants, coke ovens, sinter plant, refractory materials plant, blast furnace, steel melting shop and rolling mill, etc. There are two types of solid wastes produced in steel industry namely; ferruginous wastes and non-ferruginous wastes. Ferruginous wastes, such as iron-bearing wastes, are produced by mill scale, flue dust, steel melting shops, blast furnace slag, and other sources [3, 4].
Until the last decade, the slag, dust and sludge generated by integrated steel plants was called “waste” but now this term has been replaced by “byproduct” due to intensive re-utilization of these wastes. Discarding steel industries solid wastes in open space not only generates environmental pollution in the form of dusts and leachate but also needs huge financial responsibility.
Several studies concerning steel industry waste, especially solid waste, its management and utilization have been reported [3,4,5,6]. A few work also discussed energy recovery and energy efficiency in the steel industry by use of effective technologies [7, 8]. However, there always need to adopt novel approaches to optimize the utilization of generated byproducts which can help the iron and steel makers to solve the solid wastes problems. One among these approaches is extracting iron powder from the byproducts (wastes) obtained from integrated steel making operations. The powdered form of iron is in high demand and draws spectrum of applications [9].
Nanocomposites are one of the main types of engineering materials. They are made of at least two immiscible substances (each has its own characteristics), one of them is called the host matrix while the second is a nano-filler. Carbon black and silica are the most widely used fillers in rubber composites. However, carbon black is a non-renewable material as it is resulted from petroleum, whereas the preparation of silica involves cruel chemicals. The use of renewable materials or waste materials that possess good reinforcing, processing properties and low cost is highly preferred in the rubber industry [10,11,12]. Composites based on magnetic materials have the exclusive benefit that their properties can be modified to the requirements of specific applications. Rubber ferrite composites are ultimate for applications where specific values of relative permittivity and magnetic permeability are taken into consideration for material selection. This is true for applications concerning microwave absorption. The addition of the magnetic filler to a rubber matrix imparts magnetic properties and enhances the dielectric properties of the matrix [13]. Moreover, rubber magnetic composites papered by incorporation of strontium ferrites into non-polar and polar rubber matrices were prepared. Ferrites provide not only suitable magnetic characteristics to the rubber composites but also contribute to the improvement of the physical properties of rubber composites by improving the adhesion between the two phases [14].
Most studies, if not all investigated rubber magnetic composites prepared by incorporating laboratory prepared magnetic fillers. In the present work we consider a new approach for preparation of magnetic rubber nanocomposites by dispersing nano-waste iron powder (byproducts of integrated steel making operations) into rubber blends based on ethylene propylenediene and acrylonitrile butadiene rubber (EPDM/NBR). The rheological, mechanical, dielectric and magnetic properties of the prepared magnetic rubber nanocomposites were investigated. Also, the morphology and thermal stability were also evaluated in this study.
Experimental
Materials
-
Acrylonitrile butadiene copolymer (NBR) from Bayer AG, Germany with 32% acrylonitrile content and specific gravity 1.17 ± 0.005 at room temperature was used. EPDM, with ethylene content of 55% and density of 0.86 g cm−3, was manufactured by Esso Chemi, Germany.
-
The curing system selected contained various compounds such as mercaptobenzothiazyl disulphide (MBTS) and sulfur.
-
The activators were zinc oxide (ZnO) and stearic acid.
-
Polymerized 2,2,4-trimethyl-1,2-dihydroquinoline (TMQ) was used as an antioxidant.
-
All of the rubber ingredients were supplied by Sigma-Aldrich Co. (Munich, Germany).
-
Magnetic waste filler (MWF) shavings were collected from one of local steel’s product workshops and then sieved to get the nano-size.
Preparation of the investigated rubber nanocomposites
Rubber compounds were mixed in an open two-roll mill at room temperature. The rotors operated at a speed ratio of 1:1.4. All ingredients were mixed with rubber and finally sulfur was added. The recipe of the compounds is described in Table 1. Rubber compounds were vulcanized at 150 ± 1 °C in an electrically heated press under a pressure of about 4 Mpa to get vulcanized rubber sheets of 2 mm thickness. The vulcanization time of the sheets corresponds to optimum cure time Tc90 derived from the curing curve data.
Characterization
Transmission electron microscopy (TEM)
The particle size and electron diffraction patterns of nano-magnetic waste filler (nMWF) were examined with high-resolution transmission electron microscope JEOL JX 1230 (HRTEM).
Scanning electron microscopy (SEM)
The morphology of the NBR/EPDM blend samples was carried out using a SEM model JXA-840A (JEOL Technics) Japan. All samples were immersed in liquid nitrogen and then fractured by breaking the specimens in an appropriate size to fit in the specimen chamber. Before taking the SEM micrographs, the samples were coated with a thin layer of gold to assure electron conductivity between the samples and the specimen stage.
Rheological characteristics
The curing properties of the rubber compounds were measured by a Moving Die Rheometer (MDR one, USA) at 152 °C as per ASTM D-2084-07. From the graphs the minimum torque (ML), maximum torque (MH), optimum cure time (Tc90), scorch time Ts2) and cure rate index (CRI) {100/(Tc90−Ts2)} could be determined.
Mechanical properties
Tensile properties of rubber vulcanizates were evaluated using an Instron Universal Test Machine Model 1425, according to ASTM D412. Stress–strain curve, tensile strength, elongation at break and elastic modulus at 50, 100, 200 and 300% elongation were obtained.
Dielectric relaxation spectroscopy (DRS)
In this technique, the sample is subjected to an oscillating sinusoidal electric field. The applied voltage produces a polarization within the sample and causes a small current to flow which leads the electric field by a phase difference (tan δ).The data are presented in terms of the relative permittivity (ε′) and dielectric loss (ε″), and these are related to capacitance and conductance of the sample. The dielectric and conductivity measurements were carried out by means of high-resolution broadband impedance analyzer (SolartronTM 1260- Schlumberger Ltd, UK) interfaced with a PC as described before. The frequency range of the applied ac electric field was between 0.1 Hz and 1 MHz. Good electromagnetic shielding was implemented to the whole sample holder in order to diminish noise problems that are common especially at low frequencies. The measurements were automated by interfacing the impedance analyzer with a personal computer through a GPIB cable IEE488. A commercial interfacing and automation software LabVIEW were used for acquisition of data. Prior to the sample measurements, the calibration was performed to eliminate the effect of stray capacitance. The percent of error in permittivity ε′ and dielectric loss ε″ amounts to 1 and 3%, respectively. The temperature of the samples was controlled by a temperature regulator with Pt 100 sensor. The percent of error in temperature measurements amounts 0.5 °C. To avoid moisture, the samples were stored in desiccators in the presence of silica gel. Thereafter, the sample was transferred to the measuring cell and left with P2O5 until the measurements were carried out.
Thermogravimetric analysis (TGA)
TGA was performed using PerkinElmer (Waltham, MA) USA. The initial sample mass was about 8–10 mg; aluminum pans were used. The samples were heated from 25 to 600 °C at a heating rate of 10 °C/min under nitrogen atmosphere.
Differential scanning calorimetry
DSC 131evo (SETARAM Inc., France) was used to perform the differential scanning calorimeter analysis, Nanomaterial Investigation laboratory, Central Laboratories Network, National Research Centre (NRC), Egypt. The instrument was calibrated using the standards (Mercury, Indium, Tin, Lead, Zinc and Aluminum). Nitrogen and Helium were used as purging gases. The test was programmed, including the heating zone from − 50 °C to 100°Ca heating rate 10 °C/min. The samples were weighted in Aluminum crucible 30ul and introduced to the DSC. The thermogram results were processed using (CALISTO Data processing software v.149).
Evaluation of magnetic properties
The room temperature magnetic measurements of these NBR/EPDM/nMWF nanocomposites were carried out using Vibrating Sample Magnetometer (VSM) Lake Shore Model 7410 (USA). The magnetic parameters like Saturation magnetization (Ms), Magnetic remanence (Mr) and Coercivity (Hc) were evaluated from the hysteresis loops obtained at room temperature.
Results and discussion
Characterization of nano-waste magnetic filler (nWMF)
The XRD patterns of waste filler powder are shown at Fig. 1. Comparing diffraction lines with XRD cards illustrate that the major phase is cubic Iron (Fe), and the minor phases are cubic magnetite (Fe3O4) and hexagonal carbon (c). So our system is mostly a cubic system, which is confirmed by (TEM) photographs in Fig. 2.
Figure 2 illustrates the TEM photograph of the waste magnetic filler. From this figure, the average particle size of the particles is about 21 nm. Many agglomerates of particles in the nano-scale can also be seen.
Figure 3 shows the magnetization curve of the waste magnetic filler. It is seen that the coercivity of the filler is 44.712G which may be neglected with respect to ferromagnetism and the squareness ratio (Mr/Ms) is about 14E-3 where the saturation magnetization (Ms) is about 180 emu/g and the reminder magnetization (Mr) is about 2.62 emu/g. The concept of super paramagnetism is similar to that of ferromagnetism; the difference between the two concepts is the coherent strength of the magnetic domains. In the absence of the magnetic field, a random orientation of different magnetic domains is found but their result is zero.
From previous information we can conclude that the filler shows a super paramagnetic behavior at room temperature. As the crystal size becomes sufficiently small, the super paramagnetism appears. A single domain crystal appears when the size is less than a critical value. Magnetic crystal with a single domain has no hysteresis loop. The magnetic crystal is super paramagnetic when it has complete demagnetization as the field is stopped. At room temperature, iron oxide nanoparticles smaller than 20 nm show super paramagnetic behavior [15]. This is supported by transmission electron microscopy (TEM) result in Fig. 2.
Characterization of nanocomposites
Processability studies
The processability studies of the prepared NBR/EPDM rubber blend containing waste magnetic filler powder in nano-size (nWMF) are important since the final vulcanized product is to be molded. The processability of the rubber blend was determined by evaluating the rheological characteristics such as minimum torque ML, maximum torque MH, delta torque ΔM, scorch time Ts2, optimum cure time Tc90 and cure rate index CRI. From Table 1, it is shown that the minimum and maximum torques and delta torque (DM) increase with increasing loadings of the nWMF [16]. As the torque is directly proportional to the stiffness, the results indicated that the increase in fillers in the rubber matrix would increase the stiffness of the vulcanizates. Therefore, the incorporation of the filler in the rubber matrix could have reduced the mobility of the macromolecular chains of the compounded rubber under investigation. These observations confirm that the addition of nWMF to NBR/EPDM blend affects the processability as a reinforcement agent for the rubber blend. On the other hand, scorch time scorch time Ts2, optimum cure time Tc90 of rubber blend decreased but the cure rate index CRI increased with increasing magnetic fillers. Therefore, the changes in rheometric torque according to magnetic filler loading can be used to describe the filler-matrix interaction or reinforcement, that is reinforcement factor αf is calculated from the rheographs [17] and can be given from the following equation:
where ΔLmax (filled) and ΔLmax (gum) are the changes in torque during vulcanization for the filled and gum compounds, respectively. From Table 1, it is clear, the reinforcing factor (αf) values increased with increasing magnetic fillers concentration.
Morphology of NBR/EPDM/nWMF nanocomposites
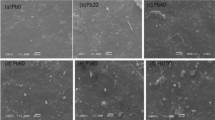
A scanning electron microscope (SEM) has been used to observe the effect of different concentrations of nano-waste magnetic filler on surface morphology for investigating the samples. Figure 4a illustrates the obtained SEM micrographs of pure NBR/EPDM (50/50) blend.
Two different phases for the individual rubbers can be seen, indicating phase separation and incompatibility of EPDM/NBR (50/50) blend [18]. Figure 4b–d illustrates the surface morphology of NBR/EPDM (50/50) blend filled with 20, 40 and 120 phr of nano-waste magnetic filler, respectively. In Fig. 4b, c, it is clear that the nano-waste magnetic filler improves the miscibility somewhat. Whereas, two distinct phases are clearly detected in the sample loaded with 120 phr nano-waste magnetic filler. On the other hand, Fig. 4a, d showed that nWMF was distributed in one phase of the blend not at the interface between the two rubbers which lead to saturation of filler in EPDM phase of NBR/EPDM blend. Later, the DSC and dielectric properties will confirm that the filler is distributed in EPDM phase.
Mechanical properties of NBR/EPDM/nWMF nanocomposites
The effect of the nano-waste magnetic filler stress–strain curves of the NBR/EPDM (50/50) blend is represented in Fig. 5.
It is evident that the presence of nano-waste magnetic filler increased the stress up to 40 phr which escorted by a minor impact on the strain of the filled blend. It is found that the higher content of the nWMF affects the stress and the extendibility of the filled blend, by reducing both of them considerably. This means lower magnetic waste filler content (≤ 40 phr) has the maximum value of stress along with attainable strain. The presence of nano-waste magnetic filler enhances the miscibility of NBR/EPDM blend (see SEM Figs. 3b, c). These observations give emphasis to the improved interaction between (host matrix and the filler) which in turn, enables bound rubber formation [19]. The values of stress and strain at rupture decreased with increasing nWMF concentration as revealed in Fig. 5a, b. This may be due to the poor dispersion of nano-magnetic waste in rubber blend (Fig. 6).
Remarkably, the values of stress at different elongation values 50, 100, 200 and 300% for NBR/EPDM/nWMF nanocomposites (Fig. 7) increased up to 40 phr and then decreased with increasing concentration of nWMF. These results confirmed the data obtained from the torque differences as tabulated in Table 1. As nano-waste magnetic filler content increases, the filler particles tend to agglomerate due to large particle–particle interactions which lead to stress failure.
Magnetic properties of NBR/EPDM/nWMF nanocomposites
The magnetization curves for all investigated nanocomposites are shown at Fig. 8a.
For all composites, the magnetization curve shows almost similar behavior as that of the waste magnetic filler, does not show a hysteresis curve; the curves are reversible with no significant remanence and no significant coercive. The variation of saturation magnetization of the composites loaded with nWMF was studied. Figure 8b shows the variation of magnetization with loading. The magnetization shows a continuous increase with nWMF loading. It increases from 0 emu/g (for 0 filler) to about 47 emu/g (for 120phr filler).
Thermogravimetry analysis (TGA)
TGA and DTA curves of 50/50 NBR/EPDM blend filled with 0, 20, 40 and 120 phr of waste magnetic filler are shown in Fig. 9a, b. Figure 9a illustrates that all samples have comparable thermogravimetric characteristics. Moreover, the degradation of all samples occurred in two stages. The first decomposition stage referred to release of the non-rubber materials. The second decomposition stage refers to rubber chain degradation. A chain separation of NBR/EPDM blend takes place as the temperature increases. Free radicals resulting from the oxidation reaction will attack the chain of NBR/EPDM blend.
AT the end of the degradation process, the mass residue is about 5.6% for the blank samples and 61.10% for NBR/EPDM blend filled with 20phr WMF. The mass residue mostly depends on the ratio of filler in the blend as listed in Table 2. Additionally, the final degradation temperatures (Tf) of blend samples increased as the volume fraction of waste magnetic filler increases. This increase in final degradation temperatures may be as a result of good interaction between filler and polymers which restricts the thermal depolymerization mechanism of the polymer and enhances thermal stability [20].
Differential scanning calorimetry (DSC) study
The DSC technique of the NBR/EPDM composites is presented in Fig. 10. Two separate glass transition temperatures Tg can be seen. The first one is at ~− 26 °C for the composites which corresponds to NBR. The other one corresponding to EPDM lies at ~ − 46 indicating immiscibility of the blend/composites [21]. This was followed immediately by the melting of the ethylene component at a temperature above 40 °C [22], which shifted to a higher temperature with the addition of nMWF.
The permittivity ε′ and dielectric loss ε″ of nanocomposites
The real and imaginary parts of permittivity (ε′, ε″) of NBR/EPDM 50/50blend are measured over a frequency range 0.1 Hz up to 10 MHz and a temperature range from 30 to 90 °C. The obtained values of ε′ and ε″ are plotted against frequency (Fig. 11a, b). This figure illustrates that ε′ values decreased as the applied frequency increased, showing anomalous dispersion. Also ε′ increased significantly at low frequencies with the increase in temperature. This noticed increase is a result of an increase in mobility of free charges as temperature increases.
From Fig. 11b, curves relating ε″ and f are broader than Debye curve, indicating the presence of more than one relaxation process. The data are fitted by a computer program based on Havriliak–Negami functions [23]. At room temperature 30 °C, in addition to the conductivity, the data were fitted by a superposition of two Havriliak–Negami functions. The low frequency absorption region that lies approximately at f = 1 Hz could be attributed to the Maxwell–Wagner effect which arises from the interfacial polarization caused by the multi-constituents of the investigated systems. The different permittivities and conductivities of the constituents of the investigated composites are considered to be the main reason for the presence of interfacial polarization. The higher absorption region with relaxation time in the order of 10−7 s could be attributed to the aggregates [17]. As the temperature increases > 40 °C, a new relaxation process in the order of 10−1 s appeared which could be attributed to the melting of the ethylene component in EPDM copolymer [23], which is agreed with DSC analysis. An example of the analysis of pure NBR/EPDM 50/50 blend at temperature 30, 70 °C is depicted in Fig. 12.
The permittivity ε′ and dielectric loss ε″ for all composites versus the applied frequency at the same range of frequency and temperature were also investigated. Examples of the obtained data are given in Fig. 13a–d for NBR/EPDM 50/50blend filled with 20 and 120 phr of nWMF. From these figures, the values of ε′ and ε″ increased by increasing nWMF concentration, which can be directly related to the increase in polarization and conductivity of the composite. However, for all samples, the curves relating ε″ and frequency were analyzed using the previous three relaxation processes in addition to the conductivity term. Examples of the analysis are illustrated in Fig. 14 for NBR/EPDM/20 phr nWMF at temperatures 30 and 90° C, respectively. From DSC analysis it is found that the melting temperature of the ethylene component in EPDM copolymer increases as the filler content increase, see Fig. 10) which is agreed with dielectric analysis in Fig. 14.
Figure 14a, b shows analysis curves for the blend containing 20phr before and after the melting temperature of the ethylene component in EPDM copolymer, respectively. In Fig. 14a the first absorption region, at f = 1 Hz, could be attributed to the Maxwell Wagner, the second absorption region, at f = 10 Hz, could be attributed to aggregates and the third one, lies approximately at f = 106 Hz could be attributed to small aggregates orientations caused by the movement of main chain [17]. In Figs. 14bfourth relaxation appears, which is attributed to the melting of the ethylene component in EPDM copolymer, this relaxation process shifted to a lower frequency with increase in filler content. This illustrates an interaction between the filler and EPDM phase, especially ethyl component. This finding not only in line with DSC analysis but also SEM analysis which illustrates that filler distributed in one phase. From previous analyses it is clear that all processes shifted to high frequency with increase in temperature, this may be as a result of mobility increment. Except Maxwell–Wagner process which approximately constant with filler content, all processes shifted to lower frequency with an increase in filler content. This because of increasing nWMF aggregates, hence relaxation time increases.
Figure 15a, b shows the effect of nano-waste magnetic filler on the permittivity ε′ and dielectric loss ε″ at 100 Hz, over the same temperature range. It is clear that is a remarkable increase in εʹ and ε″ values with the nano-waste magnetic content after 20 phr. The increase in ε′ values after this concentration may be due to raise number of dipoles. However, two abrupt increased regions appear at 20 and 40 phr WMF. This may be due to two formations of chains like aggregates of conductive filler at 20 and 40 phr, i.e., two percolation thresholds. Theoretically, the dielectric permittivity values of the composites must be decreased with increase in nWMF concentration in the post-percolation threshold region. In the post-percolation region, the composites become conductors and have a leakage current that lowers the dielectric properties of the composites. In this work, the increment in dielectric permittivity was incessant even after the first percolation threshold (at 20phr) (see Fig. 15a) and went on to another percolation at 80phr.
Electrical conductivity of NBR/EPDM/nWMF nanocomposites
The obtained conductivity (From analysis curves) of composites as a function of magnetic filler content (nWMF) over temperature range from 30° C up to 90° C are shown in Fig. 16. In our work, the difference in types of (nWMF) network in the polymer matrix at each percolation threshold caused two percolation thresholds. The two different types of networks at each percolation threshold may be attributed to the distribution of (nWMF) uniquely. The first percolation threshold (20phr) may be as a result of percolating networks of high and low density zones of (nWMF) per unit volume in the polymer matrix.
In high (nWMF) density zones, (nWMF) particles aggregated. The term “Aggregate” does not mean that (nWMF) particles contact with each other directly, a very thin rubber film separates (nWMF) particles from each other and thus can be considered as dispersion. For the second percolation threshold (80phr), (nWMF) networks were due to (nWMF) aggregates only (high-density (nWMF) zones), which resulted in a high dielectric permittivity value (11,16 at 30 and 90 °C, respectively) of the composites. The electric conductivity curve of our composites is shown in Fig. 17. This figure illustrates a wide and quasi-linear electrical conductivity curve compared with other reported curves that are usually sharp in nature [24]. In the case of a sharp conductivity curve, plateau conductivity soon after the percolation threshold is achieved, and the composites become conductive, and no more increase in the dielectric permittivity values. In our case, Post–first percolation conductivity increased gradually from 10−13 S/cm at 20 phr of (nWMF) (fc1) to 10−12 S/cm at 80 phr of (nWMF) (fc2). This behavior allows further increment in the dielectric permittivity of composites that subsequently causes another transition at 40phr. To confirm the presence of two percolations in our composites system, the constant q can be used as a testing tool in the following equation.where ρc is the volume fraction of the filler at the percolation threshold, the critical exponent (q) governs the scaling behavior in the region of ρc [25]. For one percolating system, the universal values of q are in the range of 0.8–1 [26]. From Fig. 17, according to the values of q calculated, the minor transition at 80 phr is neglected and only the single transition at 20 is considered. The q values (0.7 and 0.5) at 30 and 90 °C are not located in the universal value range of 0.8–1, which supports the rolling of two percolation thresholds.
Conclusion
The XRD patterns of (nWMF) illustrate the mostly cubic system. From the TEM photograph, the average particle size of the (nWMF) is about 21 nm. At room temperature, (nWMF) showed a super paramagnetic behavior with neglected coercivity, with respect to ferromagnetism, (44.712G) and squareness ratio (Mr/Ms) is about 14E-3.SEM of pure NBR/EPDM (50/50) blend illustrated two different phases for the individual rubbers indicating phase separation and incompatibility of the blend. While SEM of NBR/EPDM/nWMF composites showed that (nWMF) is distributed in one phase of the blend. As (nWMF) content increased (> 40) phr, the filler particles tend to agglomerate due to large particle–particle interactions which lead to stress failure. NBR/EPDM/nWMF composites show almost similar behavior as nWMF with a continuous increase in magnetization with increasing (nWMF) loading, the magnetization increased from zero to about 50 emu/g at 120 phr loading. The mass residue and the final degradation temperatures (Tf) of NBR/EPDM/nWMF composites increased as the concentration of (nWMF) filler increases. From DSC technique, two separate Tg temperatures can be seen at ~− 26 °C and ~ − 46, corresponding to NBR and EPDM respectively, indicating the immiscibility of the blend/composites the melting of the ethylene component at temperature above 40 °C [20], which shifted to higher temperature with the addition of (nMWF). For all composites, the values of ε′ and ε″ increased by increasing (nWMF) concentration. From analysis curves of ε″ before and after the melting temperature of the ethylene component in EPDM copolymer respectively, the first absorption region that lies approximately at f = 1 Hz could be attributed to the Maxwell–Wagner, the second absorption region that lies approximately at f = 10 Hz could be attributed to aggregates and the third one, lies approximately at f = 106 Hz could be associated with those orientations of small aggregates caused by the movement of the main chain. As the temperature increased, a fourth relaxation appeared which is attributed to the melting of the ethylene component in EPDM copolymer, this relaxation process shifted to a low frequency with increase in filler content. The obtained conductivity illustrated two percolation thresholds, at 20 and 80 phr. In this study, we found that the (nWMF) improved the thermal, mechanical, electrical and magnetic properties of nanocomposites so, it may be useful in the development of magnetic sensors.
References
Abdel-Shafy HI, Mansour MSM (2018) Solid waste issue: sources, composition, disposal, recycling, and valorization. Egypt J Pet 27(4):1275–1290
Allen DT, Behmanesh N (1994) Wastes as raw materials. National Academy Press, Washington, DC
Yüksel İ (2017) A review of steel slag usage in construction industry for sustainable development. Environ Dev Sustain 19(2):369–384
Guo J, Bao Y, Wang M (2018) Steel slag in China: treatment, recycling, and management. Waste Manag 78:318–330
Gupta VK, Ali I, Saini VK (2006) Adsorption of 2, 4-D and carbofuran pesticides using fertilizer and steel industry wastes. J Colloid Interface Sci 299(2):556–563
Rubio-Cintas MD, Barnett SJ, Perez-García F, Parron-Rubio ME (2019) Mechanical-strength characteristics of concrete made with stainless steel industry wastes as binders. Constr Build Mater 204:675–683
Zhang H, Wang H, Zhu X, Qiu Y-J, Li K, Chen R, Liao Q (2013) A review of waste heat recovery technologies towards molten slag in steel industry. Appl Energy 112:956–966
Worrell E, Paul B, Maarten N, Eliane B, Eric M (2010) Energy efficiency improvement and cost saving opportunities for the US iron and steel industry an ENERGY STAR (R) guide for energy and plant managers. No. LBNL-4779E. Lawrence Berkeley National Lab (LBNL), Berkeley, CA US
Eissa M, Azza A, Mohamed EF (2015) Conversion of mill scale waste into valuable products via carbothermic reduction. Journal of Metallurgy 2015
Khalf AI, Ward AA (2010) Use of rice husks as potential filler in styrene butadiene rubber/linear low density polyethylene blends in the presence of maleic anhydride. Mater Des 1980–2015 31(5):2414–2421
Ward AA, Abd-El-Messieh SL, Khalaf AI, El Nashar DA (2019) Use of natural oils as potential additives in filled rubber compounds for antistatic applications. KGK-Kautsch Gummi Kunstst 72(6):36–44
Yu P, He H, Jia Y, Tian S, Chen J, Jia D, Luo Y (2016) A comprehensive study on lignin as a green alternative of silica in natural rubber composites. Polym Testing 54:176–185
Che R, Li Y, Chen Z, Lin H (1999) Microwave absorption properties of composite fine powder synthesized by polycarbonsilane precursor/magnetic powder. J Mater Sci Lett 18(23):1963–1964
Kruželák J, Dosoudil R, Sýkora R, Hudec I (2014) Rubber composites with incorporated magnetic filler. Polimery 59(11–12):819–824
Li Q, Kartikowati CW, Horie S, Ogi T, Iwaki T, Okuyama K (2017) Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe 3 O 4 nanoparticles. Sci Rep 7(1):1–7
Ismail MN, Khalaf AI (2011) Styrene–butadiene rubber/graphite powder composites rheometrical, physicomechanical, and morphological properties. J Appl Polym Sci 120:298–304
Khalaf AI, Helaly FM, Sanaa ES (2020) Improvement properties of EPDM rubber using hybrid chitin/clay filler for industrial products. Egypt J Chem 63(1):129–143
Srivastava VK, Basak GC, Saha S, Srinivasa Rao GS, Jasra RV (2022) Epoxidized polybutadiene rubber as an effective compatibilizer for acrylonitrile butadiene rubber and polybutadiene rubber blend. J Elastom Plast 54:42–55
Ward AA, Khalf AI (2009) Influence of TESPT on the Pysico-mechanical and dielectric properties of silica filled SBR composites. KGK, Kaut Gummi Kunstst 62(12):650–656
Fu S, Sun Z, Huang P, Li Y, Ning Hu (2019) Some basic aspects of polymer nanocomposites: a critical review. Nano Mater Sci 1(1):2–30
Agarwal K, Prasad M, Chakraborty A, Vishwakarma CB, Sharma RB, Setua DK (2011) Studies on phase morphology and thermo-physical properties of nitrile rubber blends. J Therm Anal Calorim 104(3):1125–1133
Sichina WJ (2000) Characterization of EPDM elastomers using DSC. Thermal Analysis Application Note
Hammami H, Arous M, Lagache M, Kallel A (2007) Study of the interfacial MWS relaxation by dielectric spectroscopy in unidirectional PZT fibres/epoxy resin composites. J Alloy Compd 430(1–2):1–8
Mamunya Y, Matzui L, Vovchenko L, Maruzhenko O, Oliynyk V, Pusz S, Kumanek B, Szeluga U (2019) Influence of conductive nano- and microfiller distribution on electrical conductivity and EMI shielding properties of polymer/carbon composites. Compos Sci Technol 170:51–59
El-Nemr KF, Balboul MR, Ali MA (2016) Electrical and mechanical properties of manganese dioxide–magnetite-filled acrylonitrile butadiene rubber blends. J Thermoplast Compos Mater 29:704–716
Shehzad K, Hakro AA, Zeng Y, Shang-Hong Y, Xiao-Hong Y, Mumtaz M, Nadeem K, Khisro NS, Zhi-Min D (2015) Two percolation thresholds and remarkably high dielectric permittivity in pristine carbon nanotube/elastomer composites. Appl Nanosci 5:969–974
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research work was not funded.
Author information
Authors and Affiliations
Contributions
Aman I. Khalaf prepared the samples, measured, interpreted and wrote the rheological characteristics and mechanical properties. Azza A. Ward measured, interpreted and wrote the electrical, thermal properties and morphology. D Abeer A. Reffaee interpreted and wrote the data of the electrical properties and the rest of the properties, whether magnetic or thermal. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reffaee, A.A., Ward, A.A. & Khalaf, A.I. Structural, magnetic and dielectric properties of waste magnetic filler rubber nanocomposites. Polym. Bull. 81, 3081–3105 (2024). https://doi.org/10.1007/s00289-023-04835-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04835-0