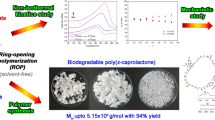
Abstract
The solvent-free ring-opening polymerization (ROP) of ε-caprolactone (ε-CL) with the tri-n-butyltin(IV) n-butoxide/aluminum(III) tri-s-butoxide mixed initiators [nBu3SnOnBu/Al(OsBu)3] was successfully studied by the non-isothermal differential scanning calorimetry for the first time. The nBu3SnOnBu/Al(OsBu)3 mixed initiators were completely dissolved in ε-CL and could be utilized to produce high molecular weight poly(ε-caprolactone) (PCL). The presence of the Al(OsBu)3 in the mixed initiators did not interfere with the initiation temperature and could increase the propagation rate of ε-CL. The activation energies (Ea) obtained from the peak methods of Kissinger and Ozawa for the ROP of ε-CL initiated by the nBu3SnOnBu/Al(OsBu)3 (1.0:2.0 mol%) (74.2–78.6 kJ/mol) were lower than nBu3SnOnBu/Al(OsBu)3 (1.0:1.0 mol%) (75.8–80.3 kJ/mol) and the single nBu3SnOnBu initiator (78.3–82.8 kJ/mol), respectively. From the proton-nuclear magnetic resonance spectroscopy (1H-NMR), the polymerization mechanism was proposed through the classic coordination–insertion mechanism that comprised: (i) the coordination of ε-CL and nBu3SnOnBu with Al(OsBu)3 and (ii) the nucleophilic attacked of n-butoxy group (–OnBu) from nBu3SnOnBu to the carbonyl carbon of ε-CL. From the small-scale synthesis (4 g) of PCL, our nBu3SnOnBu/Al(OsBu)3 mixed initiators could control the polymerization of ε-CL by adjusting the concentration of Al(OsBu)3. The number and weight average molecular weights (Mn and Mw) of PCL increased with decreasing concentration of mixed initiator. The nBu3SnOnBu/Al(OsBu)3 mixed initiators produced PCL with Mn, Mw, dispersity (Đ), and %yield in the range of 1.3 × 104–4.0 × 104 g/mol, 2.9 × 104–6.5 × 104 g/mol, 1.65–2.26 and 50–95%, respectively. The larger-scale polymerization (250 g) of ε-CL with the nBu3SnOnBu/Al(OsBu)3 mixed initiators was preliminary conducted at 150 °C for 48 h. PCL with higher Mn (7.3 × 104 g/mol) and Mw (1.2 × 105 g/mol) was synthesized. The nBu3SnOnBu/Al(OsBu)3 mixed initiators acted as an effective candidate for the production of the high molecular weight PCL via solvent-free polymerization.
Graphical abstract











Similar content being viewed by others
References
Kaihara S, Matsumura S, Mikos AG, Fisher JP (2007) Synthesis of poly(l-lactide) and polyglycolide by ring-opening polymerization. Nat Protoc 2:2767–2771
Stridsberg KM, Ryner M, Albertsson AC (2002) Controlled ring-opening polymerization: polymers with designed macromolecular architecture. Adv Polym Sci 157:41–65
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4:1466–1486
Phetsuk S, Nalampang K, Meepowpan P, Topham PD, Tighe BJ, Punyodom W (2020) Physical and thermal properties of l-lactide/ε-caprolactone copolymers: the role of microstructure design. Polym Int 69:248–256
Dash TK, Konkimalla VB (2012) Polymeric modification and its implication in drug delivery: poly-ε-caprolactone (PCL) as a model polymer. Mol Pharm 9:2365–2379
Girdthep S, Limwanich W, Punyodom W (2022) Non-isothermal cold crystallization, melting and moisture barrier properties of silver-loaded kaolinite filled poly(lactic acid) films. Mater Chem Phys 276:125227
Kowalski A, Libiszowski J, Duda A, Penczek S (2000) Polymerization of l,l-dilactide initiated by tin(II) butoxide. Macromolecules 33:1964–1971
Kricheldorf HR, Weidner SM (2021) Polymerization of l-lactide with SnCl2: a low toxic and eco-friendly catalyst. J Polym Environ 29:2504–2516
Limwanich W, Meepowpan P, Nalampang K, Kungwan N, Molloy R, Punyodom W (2015) Kinetics and thermodynamics analysis for ring-opening polymerization of ε-caprolactone initiated by tributyltin n-butoxide using differential scanning calorimetry. J Therm Anal Cal 119:567–579
Sobczak M (2012) Ring-opening polymerization of cyclic esters in the presences of choline/SnOct2 catalytic system. Polym Bull 68:2219–2228
Kricheldorf HR, Weidner SM (2021) ROP of l-lactide and ε-caprolactone catalyzed by tin(ii) and tin(iv) acetates–switching from COOH terminated linear chains to cycles. J Polym Sci 59:439–450
Punyodom W, Limwanich W, Meepowpan P, Thapsukhon B (2021) Ring-opening polymerization of ε-caprolactone initiated by tin(II) octoate/n-hexanol: DSC isoconversional kinetics analysis and polymer synthesis. Des Monomers Polym 24:89–97
Kricheldorf HR, Weidner SM (2013) High Tg copolyesters of lactide, isosorbide and isophthalic acid. Eur Polym J 49:2293–2302
Deshayes G, Mercier FAG, Degee P, Verbruggen I, Biesemans M, Willem R, Dubois P (2003) Mechanistic study of Bu2SnCl2-mediated ring-opening polymerization of ε-caprolactone by multinuclear NMR spectroscopy. Chem Eur J 9:4346–4352
Limwanich W, Meepowpan P, Kungwan N, Punyodom W (2020) Influence of butyl group of tin chloride initiators on the non-isothermal DSC ring-opening polymerization of ε-caprolactone: the studies of kinetics, mechanism and polymer synthesis. Thermochim Acta 683:178458
Silvino AC, Correa PS, Dias ML (2014) Preparation of PLLA/PDLA stereocomplexes using a novel initiator based on Mg(II) and Ti(IV) alkoxides. J Appl Polym Sci 131:40771
Silvino AC, Rodrigues ALC, Jesus KF, Dias ML (2014) Synthesis and characterization of statistical copolymers of trimethylene carbonate and l-lactide using Mg(II)/Ti(IV) mixed alkoxides as initiator system. Eur Polym J 57:66–74
Wanna N, Kraithong T, Khamnaen T, Phiriyawirut P, Charoenchaidet S, Tantirungrotechai J (2014) Aluminum- and calcium-incorporated MCM-41-type silica as supports for the immobilization of titanium(IV) isopropoxide in ring-opening polymerization of l-lactide and ε-caprolactone. Catal Commun 45:118–123
Khodabakhshi K, Gilbert M, Fathi S, Dickens P (2014) Anionic polymerization of caprolactam at the small-scale via DSC investigations: a method to be used in an additive manufacturing process. J Therm Anal Calorim 115:383–391
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Kissinger HE (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand 57:217–221
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886
Martin JL (2007) Kinetic analysis of two DSC peaks in the curing of an unsaturated polyester resin catalyzed with methylethylketone peroxide and cobalt octoate. Polym Eng Sci 47:62–70
Sriyai M, Chaiwon T, Molloy R, Meepowpan P, Punyodom W (2020) Efficiency of liquid tin(II) n-alkoxide initiators in the ring-opening polymerization of l-lactide: kinetic studies by non-isothermal differential scanning calorimetry. RSC ADV 10:43566–43578
Limwanich W, Punyodom W, Kungwan N, Meepowpan P (2015) DSC kinetics analysis for the synthesis of three-arms poly(ε-caprolactone) using aluminum tri-sec-butoxide as initiator. Int J Chem Kinet 47:734–743
Punyodom W, Thapsukhon B, Meepowpan P, Limwanich W (2022) Dibutyltin(IV) maleate as a new effective initiator for the ring-opening polymerization of ε-caprolactone: the non-isothermal kinetics, mechanism, and initiator’s performance in polymer synthesis. Polym Bull. https://doi.org/10.1007/s00289-022-04234-x
Limwanich W, Phetsuk S, Meepowpan P, Kungwan N, Punyodom W (2016) Kinetics studies of non-isothermal melt crystallization of poly(ε-caprolactone) and poly(l-lactide) Chiang Mai. J Sci 43:329–338
Damonte G, Vallin A, Fina A, Monticelli O (2021) On the development of an effective method to produce conductive PCL film. Nanomaterials 11:1385
Nakagawa S, Kadena K, Ishizone T, Nojima S, Shimizu T, Yamaguchi K, Nakahama S (2012) Crystallization behavior and crystal orientation of poly(ε-caprolactone) homopolymers confined in nanocylinders: effects of nanocylinder dimension. Macromolecules 45:1892–1900
Öztürk T, Yavuz M, Göktaş M, Hazer B (2016) One-step synthesis of triarm block copolymers by simultaneous atom transfer radical and ring-opening polymerization. Polym Bull 73:1497–1513
Punyodom W, Meepowpan P, Thapsukhon B, Dumklang M, Limwanich W (2022) Investigation of the initiating and catalytic behavior of tri-n-butyltin(IV) n-butoxide in ring-opening polymerization of ε-caprolactone and transesterification of poly(l-lactic acid) Chiang Mai. J Sci 49(1):27–38
Savaş B, Çatıker E, Öztürk T, Meyvacı E (2021) Synthesis and characterization of poly(α-methyl β-alanine)-poly(ε-caprolactone) tri arm star polymer by hydrogen transfer polymerization, ring-opening polymerization and “click” chemistry. J Polym Res 28:30
Öztürk T, Meyvacı E (2017) Synthesis and characterization poly(ϵ-caprolactone-b-ethylene glycol-b-ϵ-caprolactone) ABA type block copolymers via “Click” chemistry and ring-opening polymerization. J Macromol Sci A 54(9):575–581
Kricheldorf HR, Sumbel MV, Saunders IK (1991) Polylactones. 20. Polymerization of ε-caprolactone with tributyltin derivatives: a mechanistic study. Macromolecules 24:1944–1949
Stridsberg K, Ryner M, Albertsson AC (2000) Dihydroxy-terminated poly(l-lactide) obtained by controlled ring-opening polymerization: investigation of the polymerization mechanism. Macromolecules 33:2862–2869
Limwanich W, Meepowpan P, Sriyai M, Chaiwon T, Punyodom W (2020) Eco-friendly synthesis of biodegradable poly(ε-caprolactone) using l-lactic and glycolic acids as organic initiator. Polym Bull 78:7089–7101
Stjerndahl A, Wistrand AF, Albertsson AC (2007) Industrial utilization of tin-initiated resorbable polymers: synthesis on a large scale with a low amount of initiator residue. Macromolecules 8:937–940
Acknowledgements
This research was supported by the Program Management Unit for Human Resources & Institutional Development, Research and Innovation, Office of National Higher Education Science Research and Innovation Policy Council (NXPO) (grant number B16F640001). We also would like to thank the Center of Excellence in Materials Science and Technology, Chiang Mai University, for partially supports. In addition, the Faculty of Science and Agricultural Technology, Rajamangala University of Technology Lanna (WL) (Fundamental Fund 2023, grant number 2566FF062) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Limwanich, W., Punyodom, W. & Meepowpan, P. The role of tri-n-butyltin(IV) n-butoxide/aluminum(III) tri-s-butoxide mixed initiators in the non-isothermal ring-opening polymerization of ε-caprolactone: from small-scale to larger-scale polymerization. Polym. Bull. 81, 1159–1178 (2024). https://doi.org/10.1007/s00289-023-04764-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04764-y