Abstract
Low-molecular weight poly(ε-caprolactone), polylactides, and copolymers of ε-caprolactone and lactide were synthesized by ring-opening polymerization of cyclic monomers with choline (CHOL) as a initiator and SnOct2 as a catalyst. Polymerization was conducted in bulk (120–160 °C) with high yield. Effects of temperature, reaction time, and CHOL dosage on the polymerization process were examined. Structure of products was characterized by means of MALDI-TOF, IR, and NMR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aliphatic polyesters are probably the most important examples of biodegradable or bioresorbable polymers. Polylactide (PLA), polyglycolide (PGA), and poly(ε-caprolactone) (PCL) are regarded as a soft and hard-tissue compatible material, which has found use in resorbable sutures, drug-delivery systems, and bone graft substitutes [1–4]. For their synthesis, various cationic or anionic initiators, catalysts, and enzymes have been used [1, 5–15].
It is commonly known that the stannous octoate SnOct2 is the most often used catalyst in the ring-opening polymerization of cyclic esters. It is preferred for biomedical applications because of its low toxicity, too. The SnOct2 has been permitted as a food additive by the US FDA [1]. During the past 20 years, the mechanism of the ring-opening polymerization of cyclic esters using the SnOct2 has been the subject of much debate in the literature. It must be used together with a nucleophilic compound (generally an alcohol) to initiate the reaction if a controlled synthesis of the polymer is to be obtained [16–20].
In this article, I report the ring-opening polymerization and copolymerization of CL and rac-LA in the presence of choline (CHOL)/SnOct2 catalytic system and characterization of the resulting polyesters.
CHOL is a dietary component and found in foods as free CHOL and as esterified forms such as phosphocholine, glycerophosphocholine, sphingomyelin, and phosphatidylcholine. It functions as a precursor for acetylcholine, phospholipids, and the methyl donor betaine and is important for the structural integrity of cell membranes, methyl metabolism, cholinergic neurotransmission, transmembrane signaling, and lipid and cholesterol transport and metabolism.
Nederberg et al. have reported the novel synthesis of a “new generation” of phospholipids analogs. Their three-step synthesis has combined the use of biodegradable polyesters, such as PCL with phosphoryl choline (PC). They found that the combination of hydrophilic PC and hydrophobic polymer segments makes the materials amphiphilic [21].
The aim was to obtain a low-molecular weight polyesters, terminated by hydroxyl groups, with respect to their applications in the synthesis of prodrugs. I believed that the prepared polyesters would provide useful applications for controlled drug release.
Experimental
Materials
3,6-Dimethyl-1,4-dioxane-2,5-dione, (rac-lactide, 98%, rac-LA, Aldrich) was crystallized from a mixture of dry toluene with hexane and dried at room temperature under vacuum. ε-Caprolactone (2-Oxepanone, 99%, CL) was purchased from Aldrich. Before use, it was dried and distilled over CaH2 at reduced pressure. Choline chloride (CHOL, 98%, CA, Sigma) was dried at room temperature under vacuum for 2 h. Stannous octoate (tin (II) 2-ethylhexanoate, 95%, SnOct2, Aldrich) was used as received.
Polymerization procedure
Homo- and copolymerization of cyclic esters were carried out in the same way. Monomers (CL, rac-LA), CHOL, and SnOct2 were placed in 10 mL glass ampoules under an argon atmosphere. The reaction vessels were then left standing at the required temperature in a thermostated oil bath for the appropriate time (Tables 1, 2, 3). When the reaction was complete, the cold product was dissolved in dichloromethane, the obtained solution was washed with methanol and dilute hydrochloric acid (5% aqueous solution) under vigorous stirring. The isolated powdery or oily polymer was dried in vacuum for 48 h.
Measurements
The polymerization products were characterized by means of 1H- and 13C-NMR (Varian 300 MHz), and FTIR spectroscopy (Perkin-Elmer). The NMR spectra were recorded in CDCl3. The IR spectra were measured from KBr pellets. Relative molecular mass values and molecular mass distributions were determined using MALDI-TOF and gel permeation chromatography (GPC). The MALDI-TOF spectra were measured in the linear mode on a Kompact MALDI 4 Kratos analytical spectrometer using a nitrogen gas laser and 2-[(4-hydroxyphenyl)diazenyl] benzoic acid (HABA) as a matrix. Molecular mass values and molecular mass distributions of polymers were determined at 308 K on a Lab Alliance gel permeation chromatograph equipped with Jordi Gel DVB Mixed Bed (250 mm × 10 mm) columns and a refractive detector, using THF or chloroform as eluent (1 mL/min). The molecular mass scale was calibrated with polystyrene standards. Polymer viscosity was measured in chloroform (at 25 °C) and N,N-dimethylformamide (at 30 °C) on Stabinger Viscometer SVM 3000.
IR and NMR data
PCL: 1H-NMR (CDCl3, δ, ppm): 1.38 (2H, m, –OCH2CH2CH 2 CH2CH2C(O)O–), 1.63 (4H, m, –OCH2CH 2 CH2CH 2CH2C(O)O–), 2.27 (2H, t, –OCH2CH2CH2CH2CH 2 C(O)O–), 4.04 (2H, t, –OCH 2 CH2CH2CH2CH2C(O)O–), 13C-NMR (CDCl3, δ, ppm): 24.9 (–OCH2CH2 CH2CH2CH2C(O)O–), 25.8 (–OCH2CH2CH2 CH2CH2C(O)O–), 28.4 (–OCH2 CH2CH2CH2CH2C(O)O–), 33.8 (–OCH2CH2CH2CH2 CH2C(O)O–), 64.4 (–OCH2CH2CH2CH2CH2C(O)O–), 173.8 (–OCH2CH2CH2CH2CH2 C(O)O–); FTIR (KBr, cm−1): 2.945 (νasCH2), 2.868 (νasCH3), 1.723 (νC=O), 1.245 (νC–O).
PLA: 1H-NMR (CDCl3, δ, ppm): 5.17 (1H, q, –CH(CH3)–), 1.58 (3H, d, –CH 3 ); 13C-NMR (CDCl3, δ, ppm): 169.8 (–C(O)O–), 69.2 (–CH(CH3)–), 16.8 (–CH3); FTIR: 2997 (υasCH3), 2947 (υsCH3), 2882 (υCH), 1760 (υC=O), 1452 (δasCH3), 1348–1388 (δsCH3), 1368–1360 (δ1CH + δsCH3), 1315–1300 (δ2CH), 1270 (δCH + υCOC), 1130 (rasCH3), 1100–1090 (υsCOC), 1045 (υC–CH3), 960–950 (rCH3 + υCC), 875–860 (υC–COO), 760–740 (δC=O), 715–695 (γC=O), 515 (δ1C–CH3 + δCCO), 415 (δCCO), 350 (δ2C–CH3 + δCOC), 300–295 (δCOC + δ2C–CH3), 240 (τCC).
CL/LA copolymers: 1H-NMR (CDCl3, δ, ppm): 5.15 (1H, q, –CH(CH3)–), 4.11 (2H, t, –CH2CH 2 OC(O)–LA), 4.03 (2H, t, –CH2CH 2 OC(O)–), 2.37 (2H, t, –CH2CH2CH2CH 2 COO–LA), 2.29 (2H, t, –CH2CH 2 COO–), 1.63 (4H, m, –CH 2 CH2COO–), 1.59 (3H, d, –CH 3 ), 1.34 (2H, m, –CH2CH2CH 2 CH2CH2–).
Results and discussion
The aim of my research was to obtain low-molecular weight polyesters which can be subsequently used as polyester conjugate of drugs. Homo- and copolymers of CL and rac-LA were synthesized by ROP in the presence of SnOct2 as catalyst and CHOL as a chain control agent (Scheme 1). The polymerization reaction was carried out in different conditions by changing the reaction temperature, time, and concentration of catalyst and chain control agent (Tables 1, 2, 3).
Initially, the effectiveness of the CHOL/SnOct2 catalytic system was examined for ROP of CL. Melt polymerization was performed at 120–160 °C for 24–72 h under an argon atmosphere. The molar ratio of CL:SnOct2:CHOL was 25:0.5:1, 50:0.5:1, 100:0.5:1, or 100:1:1. The process was conducted without CHOL as initiator, too. The results of these melt polymerization runs are summarized in Table 1.
The PCL yield had tendency to decrease with the increasing CL/SnOct2/CHOL feed ratio. For PCL-2, PCL-3, and PCL-4, the yield values were 81, 77, and 73%, respectively. The reaction yield increased with the increasing temperature process, too. For PCL-2, PCL-7, and PCL-9, the yield values were 81, 85, and 93%, respectively. The reaction yield increased with the increasing reaction time, too.
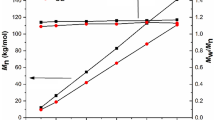
As indicated in Table 1, the M n increase with increasing M/I ratios maintaining narrow polydispersity (PD) indices, thus demonstrating that the level of polymerization control is relatively high. The PCL products were obtained with M n (from GPC) of 2100, 4100, and 7900 Da for PCL-2, PCL-3, and PCL-4, respectively. Similar differences were observed between the intrinsic viscosities (Table 1). As shown in Table 1, the PCL products were obtained with the intrinsic viscosity of 0.06, 0.08, and 0.14 dL/g for PCL-2, PCL-3, and PCL-4, respectively. Without CHOL, the M n of PCL was higher and decreased as SnOct2 was increased.
The structure of the PCL was characterized by 1H-NMR spectra as shown in Fig. 1. The results clearly indicated that typical 1H-NMR (CDCl3) chemical shifts at 1.38 (CH 2 CH2CH2COO), 1.63 (CH 2 CH2COO), 2.27 (CH 2 COO), and 4.04 (CH 2 OCO) ppm were associated with PCL.
The presence of CHOL group was confirmed by spectral analysis. The proton NMR peak at 4.3, 3.7, and 2.4 ppm was observed in all products, obtained by homopolymerization of CL and rac-LA in the presence of CHOL. The formation of the CHOL group indicates that the CHOL was incorporated into polyesters chains.
Next, the ROP of rac-LA was carried out at 120–160 °C for 24–72 h (Table 2). The molar ratio of CL:SnOct2:CHOL was 25:0.5:1, 50:0.5:1, 100:0.5:1 or 100:1:1.
The structure PLA was confirmed by spectral analysis. In 1H-NMR spectra of PLA, the main signals characteristic for repeating units appear at 1.58 (CH 3 ) and 5.17 (CHOC(O)O).
The yields of the PLA were about 21–59%. It increased with the decreasing CL/SnOct2/CHOL feed ratio and the increasing temperature process. It seems most likely that in the bulk polymerization, the decrease of reaction temperature increases the viscosity of the reaction mixture which reduces the activity of propagation species. On the other hand, higher temperature intensifies the transesterification caused by “the back-biting” process. As a result, decrease of polymer PD is observed.
The M n values of PLA determined by the GPC method are in the 1700–8100 Da range. The PD shows only small variations (between 1.2 and 1.4). As shown, the M n of PLA is dependent on the SnOct2/CHOL/rac-LA molar ratio. A similar behavior was observed for the bulk polymerization of CL. The η inh values of LA oligomers were in the range of 0.07–0.21 dL/g. Thus, the increase ratio of rac-LA to SnOct2 and CHOL led to an increase of η inh of the PLA. For example, the η inh for PLA-2, PLA-3, and PLA-4 were 0.07, 0.10, and 0.13 dL/g, respectively.
The PCL and PLA structures were elucidated by means of MALDI-TOF, too. As was shown in the MALDI-TOF mass spectrum of PLA (Fig. 2), only one series of peaks are observed. It corresponds to PLA molecules, terminated with hydroxyl groups (residual mass: 40 Da, Na+ adduct), probably. The odd number of acid m.u. shows that under the reaction conditions, the polymer chain undergoes intermolecular transesterification (leading to an exchange of segments). The M n values of PLA determined by the MALDI-TOF method are in the 1600–3200 Da range (PD indexes 1.2–1.4). The MALDI-TOF spectra of PCL contain one series of peaks, too. It is characterized by a mass increment of 114 Da, which is equal to the mass of the repeating unit in the PCL. It is assigned to PCL terminated with the hydroxyl groups (SnOct2–PCL–CHOL, residual mass: 41 Da, Na+ adduct), probably.
Next the CL was copolymerized with rac-LA in the melt at 120–140 °C for 48 h (Table 3). The molar ratio of CL/LA/SnOct2/CHOL was 25:25:0.5:1 and 50:50: 0.5:1.
The M n of copolymers determined by the GPC method is in the 3,100–5,500 Da range. All of the copolymers gave similar unimodal GPC molecular weight distributions, an example of which is shown in Fig. 3. The η inh of copolymers is in the 0.07–0.11 dL/g. The yield of copolymers was in the range of 39–67%.
Copolymer compositions were determined from the 1H-NMR spectra by taking the ratio of the peak areas corresponding to the LA –OC(O)CH(CH3)O– protons at δ = 5.0–5.2 ppm and the CL (–C(O)CH 2 CH2CH2CH2CH2O–) protons at δ = 3.9–4.1 ppm. As indicated in Table 3, the CL content in the copolymer chain of CL and LA exceeded the CL feed ratio for copolymers (amounts to 56–69 mol%).
Unfortunately, in almost all copolymers LA contents was lower than that calculated from feed ratio. It was probably due to the more reactivates of CL in the copolymerization process.
Considering the preliminary data obtained in this study, mechanism of ROP of CL and rac-LA in the presence of CHOL–SnOct2 catalytic system can be postulated as shown in Scheme 2. CHOL–SnOct2 catalytic system follows a similar reaction mechanism as alcohol–SnOct2 system, probably [16–20]. It is well known that the efficiency of SnOct2 increases when an alcohol is added as co-initiator.
The first step of the polymerization consists of the production of the active species by reacting the CHOL with the tin catalyst. Subsequently, the polymeric chain will grow by insertion of the monomer into the alkoxide bond. The initiation mechanism is thus proposed to involve the nucleophilic attack of the hydroxyl of CHOL on the carbonyl group of CL or rac-LA, which is activated by SnOct2.
Relevant kinetic, mechanistic, and toxicity studies are underway and they will be presented in the next paper.
Conclusions
In this study, a simple and efficient method of synthesis of low-molecular weight polyesters was introduced. The polyesters were synthesized by a single-step ROP of CL or rac-LA with CHOL as the co-initiator and SnOct2 as the catalyst. They can be easily produced and its yield is relatively high. It was found that increase M/I ratios enhance the reactivity of hydroxyl groups in CHOL and control the polymerization process. We hope that the obtained polyesters can find practical applications as biomaterials.
References
Labet M, Thielemans W (2009) Synthesis of polycaprolactone: a review. Chem Soc Rev 38:3484–3504. doi:10.1039/B820162P
Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM (1999) Polymeric systems for controlled drug release. Chem Rev 99:3181–3198. doi:10.1021/cr940351u
Khandare J, Minko T (2006) Polymer-drug conjugates: progress in polymeric prodrugs. Prog Polym Sci 31:359–397. doi:10.1016/j.progpolymsci.2005.09.004
Sobczak M, Witkowska E, Olędzka E, Kołodziejski WL (2008) Synthesis and structural analysis of polyester prodrugs of norfloxacin. Molecules 13:96–106. doi:10.3390/molecules13010096
Florjańczyk Z, Plichta A, Sobczak M (2006) Ring opening polymerization initiated by methylaluminoxane/AlMe3 complexes. Polymer 47:1081–1090. doi:10.1016/j.polymer.2005.11.077
Albertsson AC, Varma IV (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4(6):1466–1486. doi:10.1021/bm034247a
Duda A, Biela T, Kowalski A, Lubiszowski J (2005) Amines as (co)initiators of cyclic esters’ polymerization. Polimery 50:501–508
Martin E, Dubois P, Jerome R (2003) In situ formation of yttrium alkoxides: a versatile and efficient catalyst for the ROP of ε-caprolactone. Macromolecules 36:5934–5941
Connor EF, Nyce GW, Myers M, Moeck A, Hedrick JL (2002) First example of N-heterocyclic carbenes as catalysts for living polymerization: organocatalytic ring-opening polymerization of cyclic esters. J Am Chem Soc 124:914–915. doi:10.1021/ja0173324
Okada M Chemical syntheses of biodegradable polymers. Prog Polym Sci 27:87–133. doi: 10.1016/S0079-6700(01)00039-9
Kobayashi S, Uyama H, Kimura S (2001) Enzymatic polymerization. Chem Rev 101:3793–3818. doi:10.1021/cr990121l
Wang Ch, Li H, Zhao X (2004) Ring opening polymerization of L-lactide initiated by creatinine. Biomaterials 25:5797–5801
Sobczak M, Olędzka E, Kolodziejski WL (2008) Polymerization of cyclic esters using aminoacid initiators. J Macromol Sci Pure Appl 10:872–877. doi:10.1080/10601320802300925
Sobczak M, Kolodziejski WL (2009) Polymerization of cyclic esters initiated by carnitine and tin (II) octoate. Molecules 14(2):621–632. doi:10.3390/molecules14020621
Storey RF, Sherman JW (2002) Kinetics and mechanism of the stannous octoate-catalyzed bulk polymerization of ε-caprolactone. Macromolecules 35:1504–1512
Kowalski A, Duda A, Penczek S (2000) Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate. 3. Polymerization of L,L-dilactide. Macromolecules 33:7359–7370
Kowalski A, Duda A, Penczek S (1998) Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate, 1. Polymerization of ε-caprolactone. Macromol Rapid Comm 19:567–572. doi:10.1002/(SICI)1521-3927(19981101)19:11<567:AID-MARC567>3.3.CO;2-K
Duda A, Penczek S, Kowalski A, Libiszowski AJ (2000) Polymerizations of ε-caprolactone and L,L-dilactide initiated with stannous octoate and stannous butoxide—a comparison. Macromol Symp 153:41–50. doi:10.1002/1521-3900(200003)153:1<41:AID-MASY41>3.0.CO;2-I
Kowalski A, Duda A, Penczek S (2000) Mechanism of cyclic ester polymerization initiated with tin(II) octoate. macromolecules fitted with tin(II) alkoxide species observed directly in MALDI-TOF spectra. Macromolecules 33:689–695
Libiszowski J, Kowalski A, Duda A, Penczek S (2002) Kinetics and mechanism of cyclic esters polymerization initiated with covalent metal carboxylates, End-group studies in the model ε-caprolactone and L, L-dilactide/tin(II) and zinc octoate/butyl alcohol systems. Macromol Chem Phys 203:1694–1701. doi:10.1002/1521-3935(200207)203:10/11<1694:AID-MACP1694>3.0.CO;2-J
Nederberg F, Bowden T, Hilborn J (2004) Synthesis, characterization, and properties of phosphoryl choline functionalized poly ε-caprolactone and charged phospholipid analogues. Macromolecules 37:954–965. doi:10.1021/ma035433b
Acknowledgments
The author gratefully acknowledge the financial support of the Warsaw Medical University. The work was supported by the research program (Project MNiSW-0451/B/H03/2010/39, N N209 045139) of the Committee of Scientific Research in Poland.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sobczak, M. Ring-opening polymerization of cyclic esters in the presence of choline/SnOct2 catalytic system. Polym. Bull. 68, 2219–2228 (2012). https://doi.org/10.1007/s00289-011-0676-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-011-0676-8