Abstract
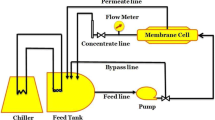
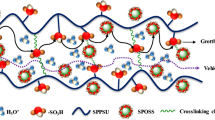
Modified attapulgite (ATP-PS) grafted with polystyrene (PS) was prepared based on Pickering emulsion polymerization. The characterization of ATP-PS was studied by transmission electronic microscopy (TEM), X-ray photoelectron spectrometer (XPS), Fourier transform infrared spectrometer (FTIR) and contact angle analysis. TEM analysis showed that the surface of ATP-PS was coated with PS organic layer; XPS analysis showed that the proportion of C element in ATP-PS was much larger than that of ATP; FTIR analysis found that ATP-PS had more PS specific functional group peaks than that of ATP; Moreover, contact angle results showed that the hydrophobicity of ATP-PS was significantly improved compared with ATP. The above characterization analysis proved that the modification of ATP-PS was successful. Moreover, polyphenylene sulfide (PPS) hydrophobic porous membrane was prepared with 1-chloronaphthalene and dibutyl phthalate as mixed diluents and ATP-PS as additives. The results showed that 0.8 wt% of ATP-PS had a great effect on the structure and performance of the membrane. With the increasing of the mass proportion of ATP-PS in the preparation of PPS base membrane, the microstructure of PPS based membrane was changed from blocky bicontinuous to spherical granular bicontinuous. When the addition amount of ATP-PS was 0.8 wt% of the total mass of PPS base membrane, PPS based membrane had strong hydrophobicity and organic solvent affinity, and its n-butanol flux could reach 106.9642 L m−2 h−1 bar−1. The rheological properties of PPS based membrane showed that ATP-PS was well compatible with the PPS, and ATP-PS was beneficial to crosslink between PPS molecular chains, thereby increasing the mechanical properties of PPS based membrane. Phase transition analysis demonstrated that ATP-PS (0.8 wt%) increased the melting temperature (2.34 °C) and crystallization temperature (2.63 °C) of PPS based membrane compared to PPS, thus allowing PPS to complete the phase transition at higher temperature interval during film formation, resulting in a stable microscopic cluster structure. Based on hydrophobic porous PPS membrane, PPS/ATP-PS/TA nanocomposites membrane with ATP-PS selective layer was prepared by co-deposition of tannic acid (TA) and ATP-PS on the surface of PPS membrane. The performance of PPS/ATP-PS/TA nanocomposites membrane was studied, which showed that the n-butanol flux of PPS/ATP-PS/TA nanocomposites membrane was 61.2913 L m−2 h−1 bar−1. More importantly, the simulated filtration performance of MB dye organic waste liquid (the solvent is n-butanol) showed that the maximum rejection rate of MB dye reached 99.82%. These results demonstrate that PPS/ATP-PS/TA nanocomposites membrane will have potential application in the filtration of organic dye wastewater.















Similar content being viewed by others
Abbreviations
- PS:
-
Polystyrene
- ATP:
-
Attapulgite
- PPS:
-
Polyphenylene sulfide
- ATP0:
-
Natural attapulgite
- ATP-A:
-
Acidized attapulgite
- ATP-K:
-
ATP-A grafted with KH570
- ATP-PS:
-
ATP-K surface grafting polymerization PS
- DBP:
-
Dibutyl phthalate
- TIPS:
-
Thermally induced phase separation
- TA:
-
Tannic acid
- DA:
-
Dopamine
- GA:
-
Gallic acid
- MB:
-
Methylene blue
- KH570:
-
γ-Methacryloxypropyltrimethoxysilane
- TEM:
-
Transmission electronic microscopy
- XPS:
-
X-ray photoelectron spectrometer
- FTIR:
-
Fourier transform infrared spectrometer
- T m :
-
Melting temperature
- T c :
-
Crystallization temperature
References
Hua M, Zhang SJ, Pan BC, Zhang WM, Lv L, Zhang QX (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331. https://doi.org/10.1016/j.jhazmat.2011.10.016
Pazdzior K, Bilinska L, Ledakowicz S (2019) A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem Eng J 376:120597. https://doi.org/10.1016/j.cej.2018.12.057
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from an artificial textile dye effluent by two agricultural waste residues, corncob and barley husk. Environ Int 28:29–33. https://doi.org/10.1016/S0160-4120(01)00131-3
Ahmad K, Nazir MA, Qureshi AK, Hussain E, Najam T, Javed MS, Shah SSA, Tufail MK, Hussain S, Khan NA, Shah H-ur-R, Ashfaq M (2020) Engineering of zirconium based metal-organic frameworks (Zr–MOFs) as efficient adsorbents. Mater Sci Eng: B 262:114766. https://doi.org/10.1016/j.mseb.2020.114766
Liang CZ, Sun SP, Li FY, Ong YK, Chung TS (2014) Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J Membr Sci 469:306–315. https://doi.org/10.1016/j.memsci.2014.06.057
Shah HUR, Ahmad K, Naseem HA, Parveen S, Ashfaq M, Rauf A, Aziz T (2021) Water stable graphene oxide metal-organic frameworks composite (ZIF-67@GO) for efficient removal of malachite green from water. Food Chem Toxicol 154:112312. https://doi.org/10.1016/j.fct.2021.112312
Ahmad K, Shah HUR, Parveen S, Aziz T, Naseem HA, Ashfaq M, Rauf A (2021) Metal organic framework (KIUB-MOF-1) as efficient adsorbent for cationic and anionic dyes from brackish water. J Mol Struct 1242:130898. https://doi.org/10.1016/j.molstruc.2021.130898
Naseem K, Ali F, Tahir MH, Afaq M, Yasir HM, Ahmed K, muteb Aljuwayid A, Habila MA (2022) Investigation of catalytic potential of sodium dodecyl sulfate stabilized silver nanoparticles for the degradation of methyl orange dye. J Mol Struct 1262:132996. https://doi.org/10.1016/j.molstruc.2022.132996
Aziz T, Nasim HA, Ahmad K, Shah H-U-R, Parveen S, Ahmad MM, Majeed H, Galal AM, Rauf A, Ashfaq M (2023) Rational synthesis, biological screening of azo derivatives of chloro-phenylcarbonyl diazenyl hydroxy dipyrimidines/thioxotetrahydropyrimidines and their metal complexes. Heliyon 9:e12492. https://doi.org/10.1016/j.heliyon.2022.e12492
Zhang PB, Tang AQ, Wang ZH, Lu JY, Zhu BK, Zhu LP (2018) Tough poly(L-DOPA)-containing double network hydrogel beads with high capacity of dye adsorption. Chin J Polym Sci 36:1251–1261. https://doi.org/10.1007/s10118-018-2163-2
Desa AL, Hairom NHH, Sidik DA, Misdan N, Yusof N, Ahmad MK, Mohammad AW (2019) A comparative study of ZnO-PVP and ZnO-PEG nanoparticles activity in membrane photocatalytic reactor (MPR) for industrial dye wastewater treatment under different membranes. J Environ Chem Eng 7:103143. https://doi.org/10.1016/j.jece.2019.103143
Liu YR, Li K, Xu WY, Du B, Wei Q, Liu B, Wei D (2020) GO/PEDOT:NaPSS modified cathode as heterogeneous electro-Fenton pretreatment and subsequently aerobic granular sludge biological degradation for dye wastewater treatment. Sci Total Environ 700:134536. https://doi.org/10.1016/j.scitotenv.2019.134536
Li Y, Wong E, Mai ZH, Van der Bruggen B (2019) Fabrication of composite polyamide/Kevlar aramid nanofiber nanofiltration membranes with high permselectivity in water desalination. J Membr Sci 592:117396. https://doi.org/10.1016/j.memsci.2019.117396
Valentino L, Matsumoto M, Dichtel WR, Marinas BJ (2017) Development and performance characterization of a polyimine covalent organic framework thin-film composite nanofiltration membrane. Environ Sci Technol 51:14352–14359. https://doi.org/10.1021/acs.est.7b04056
Abdikheibari S, Lei WW, Dumee LF, Milne N, Baskaran K (2018) Thin film nanocomposite nanofiltration membranes from amine functionalized-boron nitride/polypiperazine amide with enhanced flux and fouling resistance. J Mater Chem A 6:12066–12081. https://doi.org/10.1039/c8ta03446j
Jia TZ, Lu JP, Cheng XY, Xia QC, Cao XL, Wang Y, Xing WH, Sun SP (2019) Surface enriched sulfonated polyarylene ether benzonitrile (SPEB) that enhances heavy metal removal from polyacrylonitrile (PAN) thin-film composite nanofiltration membranes. J Membr Sci 580:214–223. https://doi.org/10.1016/j.memsci.2019.03.015
Meng J, Xie Y, Gu YH, Yan X, Chen Y, Guo XJ, Lang WZ (2021) PVDF-CaAlg nanofiltration membranes with dual thin-film-composite (TFC) structure and high permeation flux for dye removal. Sep Purif Technol 255:117739. https://doi.org/10.1016/j.seppur.2020.117739
Zhang YT, Li SH, Huang R, He J, Sun YF, Qin YY, Shen LD (2022) Stabilizing MXene-based nanofiltration membrane by forming analogous semi-interpenetrating network architecture using flexible poly(acrylic acid) for effective wastewater treatment. J Membr Sci 648:120360. https://doi.org/10.1016/j.memsci.2022.120360
Li TT, Liu L, Zhang ZM, Han ZB (2020) Preparation of nanofibrous metal-organic framework filter for rapid adsorption and selective separation of cationic dye from aqueous solution. Sep Purif Technol 237:116360. https://doi.org/10.1016/j.seppur.2019.116360
Rahimpour A, Madaeni SS (2007) Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: preparation, morphology, performance and antifouling properties. J Membr Sci 305:299–312. https://doi.org/10.1016/j.memsci.2007.08.030
Mansourizadeh A, Javadi Azad A (2014) Preparation of blend polyethersulfone/cellulose acetate/polyethylene glycol asymmetric membranes for oil–water separation. J Polym Res 21:375. https://doi.org/10.1007/s10965-014-0375-x
Fan TT, Miao JL, Li ZH, Cheng BW (2019) Bio-inspired robust superhydrophobic–superoleophilic polyphenylene sulfide membrane for efficient oil/water separation under highly acidic or alkaline conditions. J Hazard Mater 373:11–22. https://doi.org/10.1016/j.jhazmat.2019.03.008
Jung JT, Wang HH, Kim JF, Lee J, Kim JS, Drioli E, Lee YM (2018) Tailoring nonsolvent-thermally induced phase separation (N-TIPS) effect using triple spinneret to fabricate high performance PVDF hollow fiber membranes. J Membr Sci 559:117–126. https://doi.org/10.1016/j.memsci.2018.04.054
Wang XT, Li ZH, Zhang ML, Fan TT, Cheng BW (2017) Preparation of a polyphenylene sulfide membrane from a ternary polymer/solvent/non-solvent system by thermally induced phase separation. RSC Adv 7:10503–10516. https://doi.org/10.1039/c6ra28762j
Bagheripour E, Moghadassi AR, Hosseini SM, Van der Bruggen B, Parvizian F (2018) Novel composite graphene oxide/chitosan nanoplates incorporated into PES based nanofiltration membrane: chromium removal and antifouling enhancement. J Ind Eng Chem 62:311–320. https://doi.org/10.1016/j.jiec.2018.01.009
Jamil TS, Mansor ES, Abdallah H, Shaban AM (2018) Innovative high flux/low pressure blend thin film composite membranes for water softening. React Funct Polym 131:384–399. https://doi.org/10.1016/j.reactfunctpolym.2018.08.007
Wang CY, Zeng WJ, Jiang TT, Chen X, Zhang XL (2019) Incorporating attapulgite nanorods into graphene oxide nanofiltration membranes for efficient dyes wastewater treatment. Sep Purif Technol 214:21–30. https://doi.org/10.1016/j.seppur.2018.04.079
Ren L, Chen JX, Lu Q, Wang CB, Han J, Huang K, Pan XN, Wu H (2020) Construction of high selectivity and antifouling nanofiltration membrane via incorporating macrocyclic molecules into active layer. J Membr Sci 597:117641. https://doi.org/10.1016/j.memsci.2019.117641
Wu MY, Yuan JQ, Wu H, Su YL, Yang H, You XD, Zhang RN, He XY, Khan NA, Kasher R, Jiang ZY (2019) Ultrathin nanofiltration membrane with polydopamine-covalent organic framework interlayer for enhanced permeability and structural stability. J Membr Sci 576:131–141. https://doi.org/10.1016/j.memsci.2019.01.040
Li Q, Liao ZP, Fang XF, Xie J, Ni LH, Wang DP, Qi JW, Sun XY, Wang LJ, Li JS (2020) Tannic acid assisted interfacial polymerization based loose thin-film composite NF membrane for dye/salt separation. Desalination 479:114343. https://doi.org/10.1016/j.desal.2020.114343
Li YF, Su YL, Li JY, Zhao XT, Zhang RN, Fan XC, Zhu JN, Ma YY, Liu Y, Jiang ZY (2015) Preparation of thin film composite nanofiltration membrane with improved structural stability through the mediation of polydopamine. J Membr Sci 476:10–19. https://doi.org/10.1016/j.memsci.2014.11.011
Ang M, Ji YL, Huang SH, Lee KR, Lai JY (2019) A facile and versatile strategy for fabricating thin-film nanocomposite membranes with polydopamine-piperazine nanoparticles generated in situ. J Membr Sci 579:79–89. https://doi.org/10.1016/j.memsci.2019.02.064
Xu YC, Guo DX, Li T, Xiao YR, Shen LG, Li RJ, Jiao Y, Lin HJ (2020) Manipulating the mussel-inspired co-deposition of tannic acid and amine for fabrication of nanofiltration membranes with an enhanced separation performance. J Colloid Interface Sci 565:23–34. https://doi.org/10.1016/j.jcis.2020.01.004
He M, Sun HH, Sun HX, Yang XJ, Li P, Niu QJ (2019) Non-organic solvent prepared nanofiltration composite membrane from natural product tannic acid (TA) and cyclohexane-1,4-diamine (CHD). Sep Purif Technol 223:250–259. https://doi.org/10.1016/j.seppur.2019.04.064
Liu X, Xu XT, Sun J, Alsaedi A, Hayat T, Li JX, Wang XK (2018) Insight into the impact of interaction between attapulgite and graphene oxide on the adsorption of U(VI). Chem Eng J 343:217–224. https://doi.org/10.1016/j.cej.2018.02.113
Zhang WY, Qian LB, Ouyang D, Chen Y, Han L, Chen MF (2019) Effective removal of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: enhanced adsorption and crystallization. Chemosphere 221:683–692. https://doi.org/10.1016/j.chemosphere.2019.01.070
Yang FF, Song YM, Hui AP, Wang Q, Wang AQ (2022) Facile biosynthesis of ZnO/attapulgite nanocomposite for enhanced antimicrobial performance. Mater Lett 323:132549. https://doi.org/10.1016/j.matlet.2022.132549
Yu H, Zhu YF, Hui AP, Wang AQ (2022) Novel eco-friendly spherical porous adsorbent fabricated from Pickering middle internal phase emulsions for removal of Pb(II) and Cd (II). J Environ Sci 112:320–330. https://doi.org/10.1016/j.jes.2021.05.024
Wang F, Zhu YF, Wang AQ (2020) Preparation of carboxymethyl cellulose-g-poly(acrylamide)/attapulgite porous monolith with an eco-friendly pickering-MIPE template for Ce(III) and Gd(III) adsorption. Front Chem 8:00398. https://doi.org/10.3389/fchem.2020.00398
Kpogbemabou D, Lecomte-Nana G, Aimable A, Bienia M, Niknam V, Carrion C (2014) Oil-in-water Pickering emulsions stabilized by phyllosilicates at high solid content. Colloid Surf A-Physicochem Eng Asp 463:85–92. https://doi.org/10.1016/j.colsurfa.2014.09.037
Jiang LP, He CX, Fu JJ, Wang L (2018) Serviceability analysis of wood–plastic composites impregnated with paraffin-based Pickering emulsions in simulated sea water–acid rain conditions. Polym Test 70:73–80. https://doi.org/10.1016/j.polymertesting.2018.06.031
Chae HS, Piao SH, Han WJ, Choi HJ (2018) Core/shell polystyrene/magnetite hybrid nanoparticles fabricated by pickering emulsion polymerization and their magnetorheological response. Macromol Chem Phys 219:1700408. https://doi.org/10.1002/macp.201700408
Wang H, Zhao L, Song GL, Tang GY, Shi XH (2018) Organic–inorganic hybrid shell microencapsulated phase change materials prepared from SiO2/TiC-stabilized pickering emulsion polymerization. Sol Energy Mater Sol Cells 175:102–110. https://doi.org/10.1016/j.solmat.2017.09.015
Wang ZH, Qiu T, Guo LH, Ye J, He LF, Li XY (2018) Polymerization induced shaping of Pickering emulsion droplets: from simple hollow microspheres to molecularly imprinted multicore microrattles. Chem Eng J 332:409–418. https://doi.org/10.1016/j.cej.2017.09.027
He Y, Sun XK, Zhang P, Wang FF, Zhao ZZ, He CQ (2020) Cd(II) adsorption from aqueous solutions using modified attapulgite. Res Chem Intermed 46:4897–4908. https://doi.org/10.1007/s11164-020-04201-z
Zhu YF, Zhang HF, Hui AP, Ye XS, Wang AQ (2018) Fabrication of porous adsorbent via eco-friendly Pickering-MIPEs polymerization for rapid removal of Rb+ and Cs+. J Environ Chem Eng 6:849–857. https://doi.org/10.1016/j.jece.2018.01.010
He MR, Zhang SY, Su YL, Zhang RN, Liu YA, Jiang ZY (2018) Manipulating membrane surface porosity and pore size by in-situ assembly of Pluronic F127 and tannin. J Membr Sci 556:285–292. https://doi.org/10.1016/j.memsci.2018.03.087
Mamo J, Garcia-Galan MJ, Stefani M, Rodriguez-Mozaz S, Barcelo D, Monclus H, Rodriguez-Roda I, Comas J (2018) Fate of pharmaceuticals and their transformation products in integrated membrane systems for wastewater reclamation. Chem Eng J 331:450–461. https://doi.org/10.1016/j.cej.2017.08.050
Gnanasekaran L, Priya AK, Ghfar AA, Sekar K, Santhamoorthy M, Arthi M, Soto-Moscoso M (2022) The influence of heterostructured TiO2/ZnO nanomaterials for the removal of azo dye pollutant. Chemosphere 308:136161. https://doi.org/10.1016/j.chemosphere.2022.136161
Liang X, Wang PH, Wang J, Zhang YT, Wu WJ, Liu JD, Van der Bruggen B (2019) Zwitterionic functionalized MoS2 nanosheets for a novel composite membrane with effective salt/dye separation performance. J Membr Sci 573:270–279. https://doi.org/10.1016/j.memsci.2018.12.015
Lu YS, Wang AQ (2022) From structure evolution of palygorskite to functional material: a review. Microporous Mesoporous Mat 333:111765. https://doi.org/10.1016/j.micromeso.2022.111765
Xue AL, Zou XY, Zhou SY, Li MS, Zhu LW, Zhao YJ, Xing WH, Chen CL (2021) Adsorption behaviors and mechanism of heavy metals onto attapulgite functionalized by polyamine silane. J Am Ceram Soc 104:1887–1901. https://doi.org/10.1111/jace.17604
Zhang H, Wang WB, Ding JJ, Lu YS, Xu J, Wang AQ (2020) An upgraded and universal strategy to reinforce chitosan/polyvinylpyrrolidone film by incorporating active silica nanorods derived from natural palygorskite. Int J Biol Macromol 165:1276–1285. https://doi.org/10.1016/j.ijbiomac.2020.09.241
Rouhaninezhad AA, Hojati S, Masir MN (2020) Adsorption of Cr(VI) onto micro- and nanoparticles of palygorskite in aqueous solutions: effects of pH and humic acid. Ecotoxicol Environ Safe 206:111247. https://doi.org/10.1016/j.ecoenv.2020.111247
Qin L, Han J, Zhao B, Wang Y, Chen WS, Xing FT (2018) Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J Anal Appl Pyrolysis 136:132–145. https://doi.org/10.1016/j.jaap.2018.10.012
Sun SG, Ji GX, Lv YC, Liu HB, Hu T, Chen ZB, Xu SY (2021) Simultaneous recovery of ammonium and total phosphorus from toilet tail water by modified palygorskite–bentonite clay. Water Environ Res 93:1077–1086. https://doi.org/10.1002/wer.1495
El Achaby M, Qaiss A (2013) Processing and properties of polyethylene reinforced by graphene nanosheets and carbon nanotubes. Mater Des 44:81–89. https://doi.org/10.1016/j.matdes.2012.07.065
Liang JZ, Chen CY, Zou SY, Tsui CP, Tang CY, Zhang SD (2015) Melt flow behavior of polypropylene composites filled with multi-walled carbon nanotubes during extrusion. Polym Test 45:41–46. https://doi.org/10.1016/j.polymertesting.2015.05.002
Sarvi A, Sundararaj U (2014) Rheological percolation in polystyrene composites filled with polyaniline-coated multiwall carbon nanotubes. Synth Met 194:109–117. https://doi.org/10.1016/j.synthmet.2014.04.031
Gao LY, Su KM, Fan TT, Li ZH (2019) Study on the structure and properties of PPS/PCNF hybrid membranes and their applications in wastewater treatment. Polymer 176:274–282. https://doi.org/10.1016/j.polymer.2019.05.021
Gao AL, Yan YH, Li TT, Liu F (2019) Biomimetic urchin-like surface based on poly (lactic acid) membrane for robust anti-wetting and anti-bacteria properties. Mater Lett 237:240–244. https://doi.org/10.1016/j.matlet.2018.11.063
Tessmar J, Mikos A, Gopferich A (2003) The use of poly(ethylene glycol)-block-poly(lactic acid) derived copolymers for the rapid creation of biomimetic surfaces. Biomaterials 24:4475–4486. https://doi.org/10.1016/S0142-9612(03)00345-4
Acknowledgments
Financial support from the Key R&D project in Xinjiang Uygur Autonomous Region of China (2018B02016-3) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Zhen, W. Preparation, performance and structure-properties relationship of polyphenylene sulfide/ATP-PS/co-deposition of tannic acid nanocomposites membrane. Polym. Bull. 81, 969–993 (2024). https://doi.org/10.1007/s00289-023-04748-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04748-y