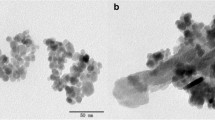
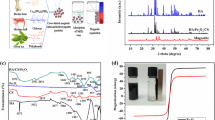
Abstract
Heavy metals and antibiotics wastewater are toxic, harmful, and have become a serious threat to human health. Here, we designed and synthesized novel biomimetic bone composites PMCH and FS@PMCH for the removal of heavy metals and antibiotics from wastewater. The pseudo-second-order and Langmuir models fitted well with the kinetic and isothermal adsorption data, respectively. Under the optimal experimental conditions (the pH of 5 and the temperature of 298.15 K), the theoretical saturated adsorption capacities for Pb (II) and TC were 377.01 mg g−1 and 99.54 mg g−1. The XPS results confirmed that the adsorption mechanism of PMCH and FS@PMCH on Pb (II) and TC was mainly electrostatic interaction and H-bond, followed by ion exchange, complexation, and pore filling. Therefore, the FS@PMCH is a potential adsorbent and purifier for Pb (II)- and TC-contaminated water.







Similar content being viewed by others
References
Rong NN, Chen CC, Ouyang KW, Zhang KJ, Wang XR, Xu ZY (2021) Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment. Sep Purif Technol 274:13. https://doi.org/10.1016/j.seppur.2021.119120
Alsaiari NS, Amari A, Katubi KM, Alzahrani FM, Ben Rebah F, Tahoon MA (2021) Innovative magnetite based polymeric nanocomposite for simultaneous removal of methyl orange and hexavalent chromium from water. Processes 9:15. https://doi.org/10.3390/pr9040576
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of china: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782. https://doi.org/10.1021/acs.est.5b00729
Dai CM, Li S, Duan YP, Leong KH, Tu Y, Zhou L (2021) Human health risk assessment of selected pharmaceuticals in the five major river basins. China Sci Total Environ 801:13. https://doi.org/10.1016/j.scitotenv.2021.149730
Cuprys A, Maletskyi Z, Rouissi T, Ratnaweera H, Brar SK, Knystautas E, Drogui P (2021) Insights into the simultaneous sorption of ciprofloxacin and heavy metals using functionalized biochar. Water 13:20. https://doi.org/10.3390/w13192768
Xiong JQ, Kurade MB, Jeon BH (2018) Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol 36:30–44. https://doi.org/10.1016/j.tibtech.2017.09.003
Ding XY, Liu YL, Chen X, Liu WJ, Li J (2021) Simultaneous removal of antibiotics and heavy metals with poly(aspartic acid)-based Fenton micromotors. Chem-Asian J 16:1930–1936. https://doi.org/10.1002/asia.202100448
Pan J, Zhu JW, Cheng FL (2021) Preparation of sodium lignosulfonate/chitosan adsorbent and application of Pb2+ treatment in water. Sustainability 13:13. https://doi.org/10.3390/su13052997
Dev VV, Baburaj G, Antony S, Arun V, Krishnan KA (2020) Zwitterion-chitosan bed for the simultaneous immobilization of Zn(II), Cd(II), Pb(II) and Cu(II) from multi-metal aqueous systems. J Cleaner Prod 255:20. https://doi.org/10.1016/j.jclepro.2020.120309
Salehi N, Moghimi A, Shahbazi H (2020) Preparation of cross-linked magnetic chitosan with methionine-glutaraldehyde for removal of heavy metals from aqueous solutions. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1753718
Peralta ME, Nistico R, Franzoso F, Magnacca G, Fernandez L, Parolo ME, Leon EG, Carlos L (2019) Highly efficient removal of heavy metals from waters by magnetic chitosan-based composite. Adsorpt J Int Adsorpt Soc 25:1337–1347. https://doi.org/10.1007/s10450-019-00096-4
Yang WX, Cheng MJ, Han Y, Luo XL, Li CH, Tang WZ, Yue TL, Li ZH (2021) Heavy metal ions’ poisoning behavior-inspired etched UiO-66/CTS aerogel for Pb(II) and Cd(II) removal from aqueous and apple juice. J Hazard Mater 401:11. https://doi.org/10.1016/j.jhazmat.2020.123318
Wang Y, Zhou RS, Wang CZ, Zhou GZ, Hua CY, Cao YY, Song ZZ (2020) Novel environmental-friendly nano-composite magnetic attapulgite functionalized by chitosan and EDTA for cadmium (II) removal. J Alloy Compd 817:13. https://doi.org/10.1016/j.jallcom.2019.153286
Ge HC, Hua TT (2016) Synthesis and characterization of poly(maleic acid)-grafted crosslinked chitosan nanomaterial with high uptake and selectivity for Hg(II) sorption. Carbohydr Polym 153:246–252. https://doi.org/10.1016/j.carbpol.2016.07.110
Liang LP, Xi FF, Tan WS, Meng X, Hu BW, Wang XK (2021) Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 3:255–281. https://doi.org/10.1007/s42773-021-00101-6
Yu SJ, Pang HW, Huang SY, Tang H, Wang SQ, Qiu MQ, Chen ZS, Yang H, Song G, Fu D, Hu BW, Wang XX (2021) Recent advances in metal-organic framework membranes for water treatment: a review. Sci Total Environ 800:22. https://doi.org/10.1016/j.scitotenv.2021.149662
Li Q, Chen ZS, Wang HH, Yang H, Wen T, Wang SQ, Hu BW, Wang XK (2021) Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci Total Environ 792:17. https://doi.org/10.1016/j.scitotenv.2021.148546
Lian ZW, Li YF, Xian HY, Ouyang XK, Lu YQ, Peng XW, Hu DL (2020) EDTA-functionalized magnetic chitosan oligosaccharide and carboxymethyl cellulose nanocomposite: synthesis, characterization, and Pb(II) adsorption performance. Int J Biol Macromol 165:591–600. https://doi.org/10.1016/j.ijbiomac.2020.09.156
Wang W, Gao M, Cao MB, Liu X, Yang HB, Li YS (2021) A series of novel carbohydrate-based carbon adsorbents were synthesized by self-propagating combustion for tetracycline removal. Bioresour Technol 332:8. https://doi.org/10.1016/j.biortech.2021.125059
Maity S, Naskar N, Jana B, Lahiri S, Ganguly J (2021) Fabrication of thiophene-chitosan hydrogel-trap for efficient immobilization of mercury (II) from aqueous environs. Carbohydr Polym 251:9. https://doi.org/10.1016/j.carbpol.2020.116999
Vatanpour V, Salehi E, Sahebjamee N, Ashrafi M (2020) Novel chitosan/polyvinyl alcohol thin membrane adsorbents modified with detonation nanodiamonds: preparation, characterization, and adsorption performance. Arab J Chem 13:1731–1740. https://doi.org/10.1016/j.arabjc.2018.01.010
Shen C, Wang YJ, Xu JH, Luo GS (2013) Chitosan supported on porous glass beads as a new green adsorbent for heavy metal recovery. Chem Eng J 229:217–224. https://doi.org/10.1016/j.cej.2013.06.003
Alves DCD, Healy B, Yu T, Breslin CB (2021) Graphene-based materials immobilized within chitosan: applications as adsorbents for the removal of aquatic pollutants. Materials 14:30. https://doi.org/10.3390/ma14133655
Varaprasad K, Nunez D, Yallapu MM, Jayaramudu T, Elgueta E, Oyarzun P (2018) Nano-hydroxyapatite polymeric hydrogels for dye removal. RSC Adv 8:18118–18127. https://doi.org/10.1039/c8ra01887a
Du JJ, Gan SC, Bian QH, Fu DH, Wei Y, Wang KQ, Lin QX, Chen WY, Huang D (2018) Preparation and characterization of porous hydroxyapatite/-cyclodextrin-based polyurethane composite scaffolds for bone tissue engineering. J Biomater Appl 33:402–409. https://doi.org/10.1177/0885328218797545
Wong SM, Zulkifli MZA, Nordin D, Teow YH (2021) Synthesis of Cellulose/Nano-hydroxyapatite composite hydrogel absorbent for removal of heavy metal ions from palm oil mill effluents. J Polym Environ 29:4106–4119. https://doi.org/10.1007/s10924-021-02183-6
Huang BY, Liu YG, Li B, Liu SB, Zeng GM, Zeng ZW, Wang XH, Ning QM, Zheng BH, Yang CP (2017) Effect of Cu(II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite. Carbohydr Polym 157:576–585. https://doi.org/10.1016/j.carbpol.2016.10.025
Zheng XY, Zheng HL, Zhou YH, Sun YJ, Zhao R, Liu YZ, Zhang SX (2019) Enhanced adsorption of Orange G from aqueous solutions by quaternary ammonium group-rich magnetic nanoparticles. Colloid Surf A-Physicochem Eng Asp 580:9. https://doi.org/10.1016/j.colsurfa.2019.123746
Roto R, Yusran Y, Kuncaka A (2016) Magnetic adsorbent of Fe3O4@SiO2 core-shell nanoparticles modified with thiol group for chloroauric ion adsorption. Appl Surf Sci 377:30–36. https://doi.org/10.1016/j.apsusc.2016.03.099
Cheng JJ, Tan GH, Li WT, Zhang HY, Wu XD, Wang ZQ, Jin YX (2016) Facile synthesis of chitosan assisted multifunctional magnetic Fe3O4@SiO2@CS@pyropheophorbide-a fluorescent nanoparticles for photodynamic therapy. New J Chem 40:8522–8534. https://doi.org/10.1039/c6nj01765g
Wang JH, Zheng SR, Shao Y, Liu JL, Xu ZY, Zhu DQ (2010) Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349:293–299. https://doi.org/10.1016/j.jcis.2010.05.010
Shao MF, Ning FY, Zhao JW, Wei M, Evans DG, Duan X (2012) Preparation of Fe3O4@SiO2@Layered double hydroxide core-shell microspheres for magnetic separation of proteins. J Am Chem Soc 134:1071–1077. https://doi.org/10.1021/ja2086323
Liu YH, Zhou YM, Yao QZ, Sun W (2015) Evaluating the performance of PEG-based scale inhibition and dispersion agent in cooling water systems. Desalin Water Treat 56:1309–1320. https://doi.org/10.1080/19443994.2014.944220
Liu YC, Yeh IC (2017) Using mixture design and neural networks to build stock selection decision support systems. Neural Comput Appl 28:521–535. https://doi.org/10.1007/s00521-015-2090-x
Lin XY, Liu J, Wan S, He X, Cui LZ, Wu GP (2019) A novel strategy for Cr(VI) removal from aqueous solution via CYPH@IL101/chitosan capsule. Int J Biol Macromol 136:35–47. https://doi.org/10.1016/j.ijbiomac.2019.05.125
Zheng LW, Gao YC, Du JH, Zhang W, Huang YJ, Wang LL, Zhao QQ, Pan XL (2020) A novel, recyclable magnetic biochar modified by chitosan-EDTA for the effective removal of Pb(II) from aqueous solution. RSC Adv 10:40196–40205. https://doi.org/10.1039/d0ra07499c
Jiang J, Long YC, Hu XJ, Hu J, Zhu MS, Zhou SQ (2020) A facile microwave-assisted synthesis of mesoporous hydroxyapatite as an efficient adsorbent for Pb2+ adsorption. J Solid State Chem 289:9. https://doi.org/10.1016/j.jssc.2020.121491
Wijesinghe W, Mantilaka M, Peiris TAN, Rajapakse RMG, Wijayantha KGU, Pitawala H, Premachandra TN, Herath H, Rajapakse R (2018) Preparation and characterization of mesoporous hydroxyapatite with non-cytotoxicity and heavy metal adsorption capacity. New J Chem 42:10271–10278. https://doi.org/10.1039/c8nj00673c
Zhang BL, Yu HY, Wang JQ, Chen X, Zhang HP, Zhang QY (2018) Fe3O4@SiO2@CCS porous magnetic microspheres as adsorbent for removal of organic dyes in aqueous phase. J Alloy Compd 735:1986–1996. https://doi.org/10.1016/j.jallcom.2017.11.349
Guo XY, Mao FF, Wang WJ, Yang Y, Bai ZM (2015) Sulfhydryl-modified Fe3O4@SiO2 core/shell nanocomposite: synthesis and toxicity assessment in vitro. ACS Appl Mater Interfaces 7:14983–14991. https://doi.org/10.1021/acsami.5b03873
Zheng XY, Zheng HL, Xiong ZK, Zhao R, Liu YZ, Zhao C, Zheng CF (2020) Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem Eng J 392:15. https://doi.org/10.1016/j.cej.2019.123706
Fan XB, Wang XH, Cai YT, Xie HH, Han SQ, Hao C (2022) Functionalized cotton charcoal/chitosan biomass-based hydrogel for capturing Pb2+, Cu2+ and MB. J Hazard Mater 423:13. https://doi.org/10.1016/j.jhazmat.2021.127191
Li SS, Wang XL, An QD, Xiao ZY, Zhai SR, Cui L, Li ZC (2020) Upon designing carboxyl methylcellulose and chitosan-derived nanostructured sorbents for efficient removal of Cd(II) and Cr(VI) from water. Int J Biol Macromol 143:640–650. https://doi.org/10.1016/j.ijbiomac.2019.12.053
Ren Y, Abbood HA, He FB, Peng H, Huang KX (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311. https://doi.org/10.1016/j.cej.2013.04.059
Le VT, Dao MU, Le HS, Tran DL, Doan VD, Nguyen HT (2020) Adsorption of Ni(II) ions by magnetic activated carbon/chitosan beads prepared from spent coffee grounds, shrimp shells and green tea extract. Environ Technol 41:2817–2832. https://doi.org/10.1080/09593330.2019.1584250
Tran HN, You SJ, Hosseini-Bandegharaei A, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116. https://doi.org/10.1016/j.watres.2017.04.014
Arshad F, Selvaraj M, Zain J, Banat F, Abu Haija M (2019) Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep Purif Technol 209:870–880. https://doi.org/10.1016/j.seppur.2018.06.035
Li M, Zhang SQ, Cui SY, Qin KX, Zhang YL, Li P, Cao QY, Xiao HN, Zeng QD (2021) Pre-grafting effect on improving adsorption efficiency of cellulose based biosorbent for Hg (II) removal from aqueous solution. Sep Purif Technol 277:12. https://doi.org/10.1016/j.seppur.2021.119493
Shan SJ, Zhang T, Wang W, Liu DM, Shi WX, Cui FY (2021) Magnetite/hydrated cerium(III) carbonate for efficient phosphate elimination from aqueous solutions and the mechanistic investigation. Chem Eng J 425:13. https://doi.org/10.1016/j.cej.2021.128894
Al-Abbad E, Alakhras F, Anastopoulos I, Das D, Al-Arfaj A, Ouerfelli N, Hosseini-Bandegharaei A (2020) Chitosan-based materials for the removal of nickel ions from aqueous solutions. Russ J Phys Chem A 94:748–755. https://doi.org/10.1134/s0036024420040032
Zhang XN, Lin XY, He Y, Chen Y, Luo XG, Shang R (2019) Study on adsorption of tetracycline by Cu-immobilized alginate adsorbent from water environment. Int J Biol Macromol 124:418–428. https://doi.org/10.1016/j.ijbiomac.2018.11.218
Alkizwini RS, Alquzweeni SS (2021) Modeling natural bentonite, thermal-modified bentonite and iron-modified bentonite with artificial neural network, sorption kinetics and sorption isotherms for simulated sorption tetracycline. Int J Environ Sci Technol 18:2633–2650. https://doi.org/10.1007/s13762-020-03004-4
Chen WQ, Xing JL, Lu ZH, Wang J, Yu SJ, Yao W, Asiri AM, Alamry KA, Wang XK, Wang SH (2018) Citrate-modified Mg–Al layered double hydroxides for efficient removal of lead from water. Environ Chem Lett 16:561–567. https://doi.org/10.1007/s10311-017-0692-5
Wen L, Zhang YM, Liu CB, Tang YH (2020) All-biomass double network gel: highly efficient removal of Pb2+ and Cd2+ in wastewater and utilization of spent adsorbents. J Polym Environ 28:2669–2680. https://doi.org/10.1007/s10924-020-01806-8
Zhu JH, Shu JC, Yue XJ, Su YP (2020) Hollow and porous octacalcium phosphate superstructures mediated by the polyelectrolyte PSS: a superior removal capacity for heavy metal and antibiotics. J Mater Sci 55:7502–7517. https://doi.org/10.1007/s10853-020-04539-0
Minale M, Gu ZL, Guadie A, Li Y, Wang Y, Meng Y, Wang XJ (2021) Hydrous manganese dioxide modified poly(sodium acrylate) hydrogel composite as a novel adsorbent for enhanced removal of tetracycline and lead from water. Chemosphere 272:12. https://doi.org/10.1016/j.chemosphere.2021.129902
He X, Zhang YX, Shen MC, Tian Y, Zheng KX, Zeng GM (2017) Vermicompost as a natural adsorbent: evaluation of simultaneous metals (Pb, Cd) and tetracycline adsorption by sewage sludge-derived vermicompost. Environ Sci Pollut Res 24:8375–8384. https://doi.org/10.1007/s11356-017-8529-0
Mandal S, Pu SY, He LL, Ma H, Hou DY (2020) Biochar induced modification of graphene oxide & nZVI and its impact on immobilization of toxic copper in soil. Environ Pollut 259:14. https://doi.org/10.1016/j.envpol.2019.113851
Jiang XY, Wang HQ, Hu EM, Lei ZW, Fan BL, Wang QL (2020) Efficient adsorption of uranium from aqueous solutions by microalgae based aerogel. Microporous Mesoporous Mater 305:8. https://doi.org/10.1016/j.micromeso.2020.110383
Ji LL, Chen W, Bi J, Zheng SR, Xu ZY, Zhu DQ, Alvarez PJ (2010) Adsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aqueous solution chemistry. Environ Toxicol Chem 29:2713–2719. https://doi.org/10.1002/etc.350
Tang L, Fang Y, Pang Y, Zeng GM, Wang JJ, Zhou YY, Deng YC, Yang GD, Cai Y, Chen J (2014) Synergistic adsorption and reduction of hexavalent chromium using highly uniform polyaniline-magnetic mesoporous silica composite. Chem Eng J 254:302–312. https://doi.org/10.1016/j.cej.2014.05.119
Wei XF, Chen SC, Rong JJ, Sui ZX, Wang SJ, Lin YB, Xiao JH, Huang DW (2021) Improving the Ca(II) adsorption of chitosan via physical and chemical modifications and charactering the structures of the calcified complexes. Polym Test 98:12. https://doi.org/10.1016/j.polymertesting.2021.107192
Acknowledgements
This work was supported by the National Key Research and Development Program of China [No.2019YFC1711300]; the Natural Science Foundation of Anhui Province [No.1808085QH289]; the Key Project of Natural Science Research of Anhui Universities [No. KJ2020A0432]; and the Quality Project of Higher Education in Anhui Province [No. 2020xfxm35].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, Z., Zhang, Y., Yan, H. et al. Adsorption of lead and tetracycline in aqueous solution by magnetic biomimetic bone composite. Polym. Bull. 81, 297–315 (2024). https://doi.org/10.1007/s00289-023-04715-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04715-7