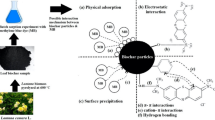
Abstract
The use of abundant and low-cost adsorbents for the removal of industrial dyes such as methylene blue (MB) and malachite green (MG) from industrial wastewater has been researched. Biochar (BC) prepared from acorn cup as an alternative adsorbent for this aim was studied. A new adsorbent was prepared using alginate-encapsulated acorn cup biochar (BC) and nano-Fe3O4 as a high-performance green absorbent. A batch-type model was used to test the removal of MB and MG from aqueous solutions by magnetic alginate-BC, m-(Alg/BC) nanocomposite as a biosorbent. Investigations were done into how initial dye concentration, adsorbent dosage, contact time, and pH variation affected adsorption removal. Optimum adsorption of MB and MG dye was observed at natural solution pH and 3 g L−1 of adsorbent dosage at 120 min of contact time. Adsorption isotherms were modeled with the Langmuir, Freundlich, Temkin, and D-R adsorption isotherms. The adsorption process is accurately described by the Langmuir isotherm model, yielding an extreme adsorption capacity of 52.63 mg g−1 for MB and 22.88 mg g−1 MG, respectively. The adsorption process followed a pseudo-second-order kinetic model, and thermodynamic studies revealed that it is an endothermic and spontaneous process. m-(Alg/BC) nanocomposite with great dye removal capacity could be used for the adsorption system.
Graphical abstract













Similar content being viewed by others
References
Banerjee S, Sharma GC, Gautam RK, Chattopadhyaya MC, Upadhyay SN, Sharma YC (2016) Removal of Malachite Green, a hazardous dye from aqueous solutions using Avena sativa (oat) hull as a potential adsorbent. J Mol Liq 213:162–172
Gündüz F, Bayrak B (2017) Biosorption of malachite green from an aqueous solution using pomegranate peel: equilibrium modelling, kinetic and thermodynamic studies. Mol Liq 243:790–798
Gürses A, Açıkyıldız M, Güneş K, Gürses MS (2016) Classification of dye and pigments. In: Sharma S (ed) Dyes and pigments. Springer, Cham, pp 31–45
Sun Y, Yu Y, Zhou S, Shah KJ, Sun W, Zhai J, Zheng H (2022) Functionalized chitosan-magnetic flocculants for heavy metal and dye removal modeled by an artificial neural network. Sep Purif Technol 282:120002
Hassan MM, Carr CM (2018) A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 209:201–219
Khorramfar S, Mahmoodi NM, Arami M, Bahrami H (2011) Oxidation of dyes from colored wastewater using activated carbon/hydrogen peroxide. Desalination 279(1–3):183–189
Thamaraiselvan C, Noel M (2015) Membrane processes for dye wastewater treatment: recent progress in fouling control. Crit Rev Environ Sci Technol 45(10):1007–1040
Alizadeh M, Jalilnejad E, Rafiee R (2019) Application of hydrogels in adsorptive removal of aqueous pollutants. Iran J Polym Sci Technol 31(6):499–518
Maslamani N, Manandhar E, Geremia DK, Logue BA (2016) ICE concentration linked with extractive stirrer (ICECLES). Anal Chim Acta 941:41–48
Ding Z, Wan Y, Hu X, Wang S, Zimmerman AR, Gao B (2016) Sorption of lead and methylene blue onto hickory biochars from different pyrolysis temperatures: importance of physicochemical properties. J Ind Eng Chem 37:261–267
Foroutan R, Mohammadi R, Ramavandi B (2019) Elimination performance of methylene blue, methyl violet, and Nile blue from aqueous media using AC/CoFe2O4 as a recyclable magnetic composite. Environ Sci Pollut Res 26(19):19523–19539
Fang J, Gao B, Zimmerman AR, Ro KS, Chen JJ (2016) A green biochar/iron oxide composite for methylene blue removal. RSC Adv 6(30):24906–24911
Premarathna KSD, Rajapaksha AU, Adassoriya N, Sarkar B, Sirimuthu NM, Cooray A, Ok YS, Vithanage M (2019) Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media. J Environ Manag 238:315–322
Yang Y, Chen N, Feng C, Li M, Gao Y (2018) Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: reaction pathway and contribution degree. Colloids Surf A Physicochem Eng Asp 556:201–209
He R, Yuan X, Huang Z, Wang H, Jiang L, Huang J, Tan M, Li H (2019) Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf A Physicochem Eng Asp 579:123642
Zhang P, O’Connor D, Wang Y, Jiang L, Xia T, Wang L, Tsang DCW, Ok YS, Hou D (2020) A green biochar/iron oxide composite for methylene blue removal. J Hazard Mater 384:121286
Wang S, Zhao M, Zhou M, Li YC, Wang J, Gao B, Sato S, Feng K, Yin W, Igalavithana AD, Oleszczuk P, Wang X, Ok YS (2019) Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: a critical review. J Hazard Mater 373:820–834
Badi MY, Azari A, Pasalari H, Esrafili A, Farzadkia M (2018) Modification of activated carbon with magnetic Fe3O4 nanoparticle composite for removal of ceftriaxone from aquatic solutions. J Mol Liq 261:146–154
Cho DW, Yoon K, Ahn Y, Sun Y, Tsang DCW, Hou D, Ok YS, Song H (2019) Fabrication and environmental applications of multifunctional mixed metal-biochar composites (MMBC) from red mud and lignin wastes. J Hazard Mater 374:412–419
Sun Y, Yu IKM, Tsang DCW, Cao X, Lin D, Wang L, Graham NJD, Alessi DS, Komárek M, Ok YS, Feng Y, Li XD (2019) Multifunctional iron-biochar 12 composites for the removal of potentially toxic elements, inherent cations, and 13 hetero-chloride from hydraulic fracturing wastewater. Environ Int 124(14):521–532
Kalia S, Kango S, Kumar A, Haldorai Y, Kumari B, Kumar R (2014) Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym Sci 292(9):2025–2052
Bhushan B, Gupta V, Kotnala S (2020) Development of magnetic-biochar nano-composite: assessment of its physico-chemical properties. Mater Today: Proceed 26:3271–3274
Nouri N, Khorram P, Duman O, Sibel T, Hassan S (2020) Overview of nanosorbents used in solid phase extraction techniques for the monitoring of emerging organic contaminants in water and wastewater samples. Trends Environ Anal Chem 25:e00081
Jawad AH, Abdulhameed AS, Selvasembian R, ALOthman ZA, Wilson LD (2022) Magnetic biohybrid chitosan-ethylene glycol diglycidyl ether/magnesium oxide/Fe3O4 nanocomposite for textile dye removal: Box-Behnken design optimization and mechanism study. J Polym Res 29(5):1–15
Jawad AH, Hameed BH, Abdulhameed AS (2022) Synthesis of biohybrid magnetic chitosan-polyvinyl alcohol/MgO nanocomposite blend for remazol brilliant blue R dye adsorption: solo and collective parametric optimization. Polym Bull. https://doi.org/10.1007/s00289-022-04294-z
Wang B, Gao B, Fang J (2017) Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol 47(22):2158–2207
Lin Y, Sun Y, Dai Y, Sun W, Zhu X, Liu H, Wang X (2020) A “signal-on” chemiluminescence biosensor for thrombin detection based on DNA functionalized magnetic sodium alginate hydrogel and metalloporphyrinic metal-organic framework nanosheets. Talanta 207:120300
Qamar SA, Qamar M, Basharat A, Bilal M, Cheng H, Iqbal HM (2022) Alginate-based nano-adsorbent materials—Bioinspired solution to mitigate hazardous environmental pollutants. Chemosphere 288:132618
Sun L, Wan S, Yuan D, Yu Z (2019) Adsorption of nitroimidazole antibiotics from aqueous solutions on self-shaping porous biomass carbon foam pellets derived from Vallisneria natans waste as a new adsorbent. Sci Total Environ 664:24–36
Parlayıcı Ş, Pehlivan E (2021) Biosorption of methylene blue and malachite green on biodegradable magnetic Cortaderia selloana flower spikes: modeling and equilibrium study. Int J Phytoremediation 23(1):26–40
Zhang MM, Liu YG, Li TT, Xu WH, Zheng BH, Tan XF, Wang FY, Wang SF (2015) Chitosan modification of magnetic biochar produced from Eichhornia crassipes for enhanced sorption of Cr (VI) from aqueous solution. RSC Adv 5(58):46955–46964
Fang Q, Chen B, Lin Y, Guan Y (2014) Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ Sci Technol 48(1):279–288
Zornoza R, Moreno-Barriga F, Acosta JA, Muñoz MA, Faz A (2016) Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 144:122–130
Nikoofar K, Arian Z, Djahaniani H (2022) Novel AlCl3@ nano Fe3O4-SiO2: a benign multi-layer magnetite nanocatalyst for the three-component one-pot synthesis of spiro[benzochromeno[2,3-d]pyrimidin-indolines]. Inorg Nano-Metal Chem 52(3):365–374
Wang Y, Zhang Y, Li S, Zhong W, Wei W (2018) Enhanced methylene blue adsorption onto activated reed-derived biochar by tannic acid. J Mol Liq 268:658–666
Abdelwahab MS, El Halfawy NM, El-Naggar MY (2022) Lead adsorption and antibacterial activity using modified magnetic biochar/sodium alginate nanocomposite. Int J Biol Macromol 206:730–739
Yu C, Wang M, Dong X, Shi Z, Zhang X, Lin Q (2017) Removal of Cu (ii) from aqueous solution using Fe3O4–alginate modified biochar microspheres. RSC Adv 7(84):53135–53144
Pi Z, Li X, Wang D, Xu Q, Tao Z, Huang X, Yao F, Wu Y, He L, Yang Q (2019) Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: Performance and degradation pathway. J Clean Prod 235:1103–1115
Pandi K, Viswanathan N (2015) Synthesis of alginate beads filled with nanohydroxyapatite: an efficient approach for fluoride sorption. J Appl Polym Sci 132(19):41937
Kim H, Watthanaphanit A, Saito N (2017) Simple solution plasma synthesis of hierarchical nanoporous MnO2 for organic dye removal. ACS Sustain Chem Eng 5(7):5842–5851
Ahmad MA, Alrozi R (2011) Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem Eng J 171(2):510–516
Nidheesh PV, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227
Liu S, Li J, Xu S, Wang M, Zhang Y, Xue X (2019) A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour Technol 282:48–55
Sarkar S, Tiwari N, Basu A, Behera M, Das B, Chakrabortty S, Sanjay K, Suar M, Adhya TK, Banerjee SS, Tripathy SK (2021) Sorptive removal of malachite green from aqueous solution by magnetite/coir pith supported sodium alginate beads: kinetics, isotherms, thermodynamics and parametric optimization. Environ Technol Innov 24:101818
Wang B, Gao B, Wan Y (2019) Comparative study of calcium alginate, ball-milled biochar, and their composites on aqueous methylene blue adsorption. Environ Sci Pollut Res 26(12):11535–11541
Othman I, Abu Haija M, Kannan P, Banat F (2020) Adsorptive removal of methylene blue from water using high-performance alginate-based beads. Water Air Soil Pollut 231(8):1–16
Jawad AH, Abdulhameed AS, Surip SN, Sabar S (2020) Adsorptive performance of carbon modified chitosan biopolymer for cationic dye removal: kinetic, isotherm, thermodynamic, and mechanism study. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1807966
Foroutan R, Peighambardoust SJ, Hemmati S, Khatooni H, Ramavandi B (2021) Preparation of clinoptilolite/starch/CoFe2O4 magnetic nanocomposite powder and its elimination properties for cationic dyes from water and wastewater. Int J Biol Macromol 189:432–442
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57(1):385–470
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Proc Acad Sci USSR Phys Chem Sect 55:331
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Scatchard GD (1949) The attractions of proteins for small molecules and ions. Ann NY Acad Sci 51:660–672
Biswas S, Mohapatra SS, Kumari U, Meikap BC, Sen TK (2020) Batch and continuous closed circuit semi-fluidized bed operation: Removal of MB dye using sugarcane bagasse biochar and alginate composite adsorbents. J Environ Chem Eng 8(1):103637
Radoor S, Karayil J, Jayakumar A, Parameswaranpillai J, Siengchin S (2021) An efficient removal of malachite green dye from aqueous environment using ZSM-5 zeolite/polyvinyl alcohol/carboxymethyl cellulose/sodium alginate bio composite. J Polym Environ 29(7):2126–2139
Djilani C, Zaghdoudi R, Djazi F, Bouchekima B, Lallam A, Modarressi A, Rogalski M (2015) Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. Taiwan Inst Chem Eng 53:112–121
Mustafa I (2019) Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchemical J 144:397–402
Gautam RK, Rawat V, Banerjee S, Sanroman MA, Soni S, Singh SK, Chattopadhyaya MC (2015) Synthesis of bimetallic Fe–Zn nanoparticles and its application towards adsorptive removal of carcinogenic dye malachite green and Congo red in water. J Mol Liq 212:227–236
Iqbal MJ, Ashiq MN (2007) Adsorption of dyes from aqueous solutions on activated charcoal. J Hazard Mater 139(1):57–66
Shah I, Adnan R, Ngah WSW, Mohamed N, Taufiq-Yap YH (2014) A new insight to the physical interpretation of activated carbon and iron doped carbon material: Sorption affinity towards organic dye. Bioresour Technol 160:52–56
Sharma YC (2010) Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J Chem Eng Data 55(1):435–439
Jawad AH, Abdulhameed AS, Hanafiah MAKM, ALOthman ZA, Khan MR, Surip SN (2021) Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: Modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J Chem Eng 38(7):1499–1509
Jawad AH, Abdulhameed AS, Bahrudin NN, Hum NNMF, Surip SN, Syed-Hassan SSA, Yousif E, Sabar S (2021) Microporous activated carbon developed from KOH activated biomass waste: surface mechanistic study of methylene blue dye adsorption. Water Sci Technol 84(8):1858–1872
Jawad AH, Abdulhameed AS, Kashi E, Yaseen ZM, ALOthman ZA, Khan MR (2022) Cross-linked chitosan-glyoxal/kaolin clay composite: parametric optimization for color removal and COD reduction of remazol brilliant blue R dye. J Polym Environ 30(1):164–178
Peighambardoust SJ, Aghamohammadi-Bavil O, Foroutan R, Arsalani N (2020) Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int J Biol Macromol 159:1122–1131
Pehlivan E, Parlayıcı Ş (2021) Fabrication of a novel biopolymer-based nanocomposite (nanoTiO2-chitosan-plum kernel shell) and adsorption of cationic dyes. J Chem Technol Biotechnol 96(12):3378–3387
Yıldırım GM, Bayrak B (2021) The synthesis of biochar-supported nano zero-valent iron composite and its adsorption performance in removal of malachite green. Biomass Convers Biorefinery 10:1–13
Gao S, Shi Y, Zhang S, Jiang K, Yang S, Li Z, Takayama-Muromachi E (2008) Biopolymer-assisted green synthesis of iron oxide nanoparticles and their magnetic properties. J Phys Chem C 112(28):10398–10401
Gupta VK, Agarwal S, Saleh TA (2011) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45(6):2207–2212
Abkenar SD, Khoobi M, Tarasi R, Hosseini M, Shafiee A, Ganjali MR (2015) Fast removal of methylene blue from aqueous solution using magnetic-modified Fe3O4 nanoparticles. J Environ Eng 141(1):04014049
Lei C, Wen F, Chen J, Chen W, Huang Y, Wang B (2021) Mussel-inspired synthesis of magnetic carboxymethyl chitosan aerogel for removal cationic and anionic dyes from aqueous solution. Polymer 213:123316
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parlayıcı, Ş., Pehlivan, E. An ecologically sustainable specific method using new magnetic alginate-biochar from acorn cups (Quercus coccifera L.) for decolorization of dyes. Polym. Bull. 80, 11167–11191 (2023). https://doi.org/10.1007/s00289-022-04609-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04609-0