Abstract
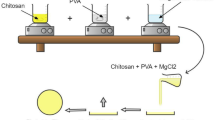
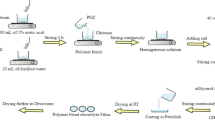
The effect of different concentrations of agarose gel electrolyte in a low-cost fabrication of DSSC system was investigated. The modified DSSC was fabricated by a sandwiched method using ITO glass plates as substrates. TiO2-Graphene-coated glass plate was used as the working electrode while PANI-GO-coated glass plate works as a counter electrode, respectively. Both electrodes were separated by agarose gel mixture with KI solution as gel biopolymer electrolyte. The FTIR result showed that peaks of agarose are well observed in the KI-agarose spectra. In the XRD analysis, the combination of KI-agarose has reduced the crystallinity of agarose which was good for ionic conductivity value. The addition of agarose in KI solution also produced a fine channel to facilitate the ionic transfer in electrolyte. The behaviour of electrochemical properties of the system was observed using AC impedance spectroscopy based on the Nyquist plot. From the plot, three semicircles were observed as the responses at different frequencies. It was observed that the 5 wt% agarose electrolyte in DSSC system was selected as the optimum loading exhibiting the lowest resistivity ensuring the faster electron transfer and giving the ionic conductivity of 9.04 × 10−1 S/cm. The ionic conductivity of the system was dropped at 2.21 × 10−1 S/cm as the percentage of agarose was increase to 6 wt%.







Similar content being viewed by others
References
Sharma S, Siwach B, Ghoshal SK, Mohan D (2015) Dye sensitized solar cells: from genesis to recent drifts. Renew Sustain Energy Rev 70:529–537. https://doi.org/10.1016/j.rser.2016.11.136
Oktariza LG, Yuliarto B, Suyatman (2018) Performance of dye sensitized solar cells (DSSC) using syngonium podophyllum schott as natural dye and counter electrode. AIP Conf Proc. https://doi.org/10.1063/1.5034553
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346):737–740. https://doi.org/10.1038/353737a0
Zulkifli AM et al (2020) Characteristics of dye-sensitized solar cell assembled from modified chitosan-based gel polymer electrolytes incorporated with potassium iodide. Molecules 25:18. https://doi.org/10.3390/molecules25184115
Qohhar R, Supriyanto E, Sujito Nugroho AT, Subekti A (2020) Photoanode nanostructure optimization in dye-sensitized solar cell. AIP Conf Proc. https://doi.org/10.1063/5.0015224
Sadikin SN, Rahman MYA, Umar AA, Aziz THT (2019) Improvement of dye-sensitized solar cell performance by utilizing graphene-coated TiO2 films photoanode. Superlattices Microstruct 128:92–98. https://doi.org/10.1016/j.spmi.2019.01.014
Lue SJ, Wu YL, Tung YL, Shih CM, Wang YC, Li JR (2015) Functional titanium oxide nano-particles as electron lifetime, electrical conductance enhancer, and long-term performance booster in quasi-solid-state electrolyte for dye-sensitized solar cells. J Power Sources 274:1283–1291. https://doi.org/10.1016/j.jpowsour.2014.10.194
Sottomesso P (2020) Fo r P ee r R ev Fo r R. Jdr 2001:3–29
Applications DSC, Nagaraj P, Sasidharan A, David V, Sambandam A (2017) Effect of cross-linking on the performances of starch-based biopolymer as gel electrolyte for. Polymers. https://doi.org/10.3390/polym9120667
Ngai KS, Ramesh S, Ramesh K, Juan JC (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics (Kiel) 22(8):1259–1279. https://doi.org/10.1007/s11581-016-1756-4
Singh R, Bhattacharya B, Tomar SK, Singh V, Singh PK (2017) Electrical, optical and electrophotochemical studies on agarose based biopolymer electrolyte towards dye sensitized solar cell application. Meas J Int Meas Confed 102:214–219. https://doi.org/10.1016/j.measurement.2017.02.014
Mohamad AA (2019) Physical properties of quasi-solid-state polymer electrolytes for dye-sensitised solar cells: a characterisation review. Sol Energy 190:434–452. https://doi.org/10.1016/j.solener.2019.08.016
Menzel J, Frąckowiak E, Fic K (2020) Agar-based aqueous electrolytes for electrochemical capacitors with reduced self-discharge. Electrochim Acta. https://doi.org/10.1016/j.electacta.2019.135435
Yan T, Zou Y, Zhang X, Li D, Guo X, Yang D (2021) Hydrogen bond interpenetrated agarose/pva network: a highly ionic conductive and flame-retardant gel polymer electrolyte. ACS Appl Mater Interfaces 13(8):9856–9864. https://doi.org/10.1021/acsami.0c20702
Cao D et al (2021) Room-temperature preparation of TiO2/graphene composite photoanodes for efficient dye-sensitized solar cells. J Colloid Interface Sci 586:326–334. https://doi.org/10.1016/j.jcis.2020.10.096
Yu Y, Wen W, Qian XY, Bin Liu J, Wu JM (2016) UV and visible light photocatalytic activity of Au/TiO 2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci Rep 7(1–13):2017. https://doi.org/10.1038/srep41253
Timoumi A, Alamri SN, Alamri H (2018) The development of TiO2-graphene oxide nano composite thin films for solar cells. Results Phys 11(2017):46–51. https://doi.org/10.1016/j.rinp.2018.06.017
Kazmi SA, Hameed S, Ahmed AS, Arshad M, Azam A (2017) Electrical and optical properties of graphene-TiO2nanocomposite and its applications in dye sensitized solar cells (DSSC). J Alloys Compd 691:659–665. https://doi.org/10.1016/j.jallcom.2016.08.319
Zhang H, Wang W, Liu H, Wang R, Chen Y, Wang Z (2014) Effects of TiO2 film thickness on photovoltaic properties of dye-sensitized solar cell and its enhanced performance by graphene combination. Mater Res Bull 49(1):126–131. https://doi.org/10.1016/j.materresbull.2013.08.058
Akilimali R et al (2018) Hybrid TiO2-Graphene nanoribbon photoanodes to improve the photoconversion efficiency of dye sensitized solar cells. J Power Sources 396(June):566–573. https://doi.org/10.1016/j.jpowsour.2018.06.044
Norhisamudin NA et al (2020) The efficiency effect of dye sensitized solar cell using different ratio of organic polymer doped titanium dioxide at different annealing process temperature. AIP Conf Proc. https://doi.org/10.1063/1.5142144
Lemos HG et al (2020) Water-dispersible polyaniline/graphene oxide counter electrodes for dye-sensitized solar cells: influence of synthesis route on the device performance. Sol Energy 207(June):1202–1213. https://doi.org/10.1016/j.solener.2020.07.021
NM Mohamed, MU Shahid, and BS Mahinder, (2018) Polyaniline (PANI)/ reduced graphene oxide (rGO) composite as a counter electrode for dye solar cells . Polyaniline (PANI)/reduced graphene oxide (rGO) composite as a counter electrode for dye solar cells.
Teo LP, Tiong TS, Buraidah MH, Arof AK (2018) Effect of lithium iodide on the performance of dye sensitized solar cells (DSSC) using poly(ethylene oxide) (PEO)/poly(vinyl alcohol) (PVA) based gel polymer electrolytes. Opt Mater (Amst) 85:531–537. https://doi.org/10.1016/j.optmat.2018.09.026
Xu P, Tang Q, Chen H, He B (2014) Insights of close contact between polyaniline and FTO substrate for enhanced photovoltaic performances of dye-sensitized solar cells. Electrochim Acta 125:163–169. https://doi.org/10.1016/j.electacta.2014.01.107
Yilmaz Erdogan P, Zengin H, Yavuz A (2020) Growth and cycling of polyaniline electrode in a deep eutectic solvent: a new electrolyte for supercapacitor applications. Solid State Ionics 352:115362. https://doi.org/10.1016/j.ssi.2020.115362
Eftekhari A, Li L, Yang Y (2017) Polyaniline supercapacitors. J Power Sources 347:86–107. https://doi.org/10.1016/j.jpowsour.2017.02.054
Eltayeb NE, Khan A (2020) Preparation and properties of newly synthesized Polyaniline@Graphene oxide/Ag nanocomposite for highly selective sensor application. J Mater Res Technol 9(5):10459–10467. https://doi.org/10.1016/j.jmrt.2020.07.031
Grätzel M (2005) Solar energy conversion by dye-sensitized photovoltaic cells. Inorg Chem 44(20):6841–6851. https://doi.org/10.1021/ic0508371
Pratiwi DD, Nurosyid Kusumandari F, Supriyanto A, Suryana R (2017) Performance improvement of dye-sensitized solar cells (DSSC) by using dyes mixture from chlorophyll and anthocyanin. J Phy Conf Ser 909:1. https://doi.org/10.1088/1742-6596/909/1/012025
Singh R, Singh PK, Singh V, Bhattacharya B (2017) Agarose biopolymer electrolytes: ion conduction mechanism and dielectric studies. Cellul Chem Technol 51(9–10):949–955
Rahmawati R, Suendo V, Hidayat R (2018) Reduced graphene oxide/polyaniline nanocomposite as efficient counter electrode for dye sensitized solar cells. IOP Conf Ser Mater Sci Eng 384:1. https://doi.org/10.1088/1757-899X/384/1/012040
Yang Y, Hu H, Zhou CH, Xu S, Sebo B, Zhao XZ (2011) Novel agarose polymer electrolyte for quasi-solid state dye-sensitized solar cell. J Power Sources 196(4):2410–2415. https://doi.org/10.1016/j.jpowsour.2010.10.067
Singh R, Jadhav NA, Majumder S, Bhattacharya B, Singh PK (2013) Novel biopolymer gel electrolyte for dye-sensitized solar cell application. Carbohydr Polym 91(2):682–685. https://doi.org/10.1016/j.carbpol.2012.08.055
Shih CM et al (2018) Ionic transport and interfacial interaction of iodide/iodine redox mechanism in agarose electrolyte containing colloidal titanium dioxide nanoparticles. J Photochem Photobiol A Chem 356:565–572. https://doi.org/10.1016/j.jphotochem.2018.01.034
R Singh, PK Singh, V Singh, and B Bhattacharya, (2017) Agarose biopolymer electrolytes: ion conduction mechanism and dielectric studies
Sharma K, Sharma V, Sharma SS (2018) Dye-Sensitized Solar Cells: fundamentals and Current Status. Nanoscale Res L Springer New York LLC. https://doi.org/10.1186/s11671-018-2760-6
Selvanathan V et al (2020) Organosoluble starch derivative as quasi-solid electrolytes in DSSC: unravelling the synergy between electrolyte rheology and photovoltaic properties. Sol Energy 197:144–153. https://doi.org/10.1016/j.solener.2019.12.074
St-Onge V, Cui M, Rochon S, Daigle JC, Claverie JP (2021) Reducing crystallinity in solid polymer electrolytes for lithium-metal batteries via statistical copolymerization. Commun Mater 2(1):1–11. https://doi.org/10.1038/s43246-021-00187-2
Singh R, Polu AR, Bhattacharya B, Rhee HW, Varlikli C, Singh PK (2016) Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew Sustain Energy Rev Elsevier Ltd 65:1098–1117. https://doi.org/10.1016/j.rser.2016.06.026
Bidikoudi M, Perganti D, Karagianni CS, Falaras P (2015) Solidification of ionic liquid redox electrolytes using agarose biopolymer for highly performing dye-sensitized solar cells. Electrochim Acta 179:228–236. https://doi.org/10.1016/j.electacta.2015.02.122
Sun P et al (2021) High-strength agarose gel electrolyte enables long-endurance wearable Al-air batteries with greatly suppressed self-corrosion. Energy Storage Mater 34:427–435. https://doi.org/10.1016/j.ensm.2020.10.009
Nagaraj P, Sasidharan A, David V, Sambandam A (2017) Effect of Cross-Linking on the Performances of Starch-Based Biopolymer as Gel Electrolyte for Dye-Sensitized Solar Cell Applications. Polymers (Basel) 9(12):667. https://doi.org/10.3390/polym9120667
Mustafa MN, Shafie S, Wahid MH, Sulaiman Y (2019) Preparation of TiO2 compact layer by heat treatment of electrospun TiO2 composite for dye-sensitized solar cells. Thin Solid Films 693(June):2020. https://doi.org/10.1016/j.tsf.2019.137699
El-Dairi M, House RJ (2019) Optic nerve hypoplasia. Handb Pediatr Retinal OCT Eye-Brain Connect. https://doi.org/10.1016/B978-0-323-60984-5.00062-7
Acknowledgements
The authors would like to thank the Faculty of Chemical Engineering Technology, University of Malaysia Perlis, for financial support by the Ministry of Higher Education Commission (MOHE) under the research project grant (Ref: FRGS/1/2019/STG01/UNIMAP/02/2) and an appreciation towards the Faculty of Applied Science, University of Technology MARA (UiTM), Arau, for the laboratory equipment throughout the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badri, A., Razak, S., Nawawi, W.I. et al. Synergistic effect of agarose biopolymer gel electrolyte with modified TiO2 for low-cost electrochemical device applications. Polym. Bull. 80, 9437–9450 (2023). https://doi.org/10.1007/s00289-022-04524-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04524-4