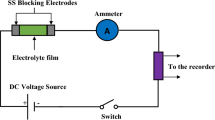
Abstract
In this research, a new system for electrical double-layer capacitors (EDLCs) is introduced that employs biopolymer blend electrolytes incorporating glycerol as a plasticizer. To prepare the films, the solution cast technique was employed to produce sodium-conducting plasticized chitosan:poly(oxazoline) (CH:POZ) systems. Sodium bromide (NaBr) salt was used as a sodium ion provider along with various concentrations of plasticizer to enhance the conduction mechanism. Using electrochemical impedance spectroscopy (EIS), the conductivity, diffusion coefficient, mobility, and number density of ions were determined via simulated EIS results. The results showed that the maximum conductivity of 8.27 × 10−4 S/cm was achieved when 45 wt.% of glycerol was added. The study also investigated the effect of plasticizers on the relaxation time for sodium conduction and the electrochemical behavior of the samples. The superior performance of the system was further confirmed by electrochemical measurements such as linear sweep voltammetry (LSV) and cyclic voltammetry (CV).












Similar content being viewed by others
Data Availability
Not applicable.
References
Jung YH et al (2015) High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat Commun 6:1–11
Hao Z et al (2020) Nanochannels regulating ionic transport for boosting electrochemical energy storage and conversion: a review. Nanoscale 12:15923–15943
Yu G, Xie X, Pan L, Bao Z, Cui Y (2013) Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2:213–234
Di Noto V, Lavina S, Giffin GA, Negro E, Scrosati B (2011) Polymer electrolytes: present, past and future. Electrochim Acta 57:4–13. https://doi.org/10.1016/j.electacta.2011.08.048
Itoh T, Mitsuda Y, Ebina T, Uno T, Kubo M (2009) Solid polymer electrolytes composed of polyanionic lithium salts and polyethers. J Power Sources 189:531–535
Samsudin AS, Khairul WM, Isa MIN (2012) Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J Non Cryst Solids 358:1104–1112. https://doi.org/10.1016/j.jnoncrysol.2012.02.004
Monisha S, Mathavan T, Selvasekarapandian S, Benial AMF, latha MP, (2017) Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics (Kiel) 23:2697–706. https://doi.org/10.1007/s11581-016-1886-8
Pandey GP, Kumar Y, Hashmi SA (2010) Ionic liquid incorporated polymer electrolytes for supercapacitor application. Indian J Chem 49:743–751
Hema M, Selvasekerapandian S, Hirankumar G, Sakunthala A, Arunkumar D, Nithya H (2009) Structural and thermal studies of PVA:NH4I. J Phys Chem Solids 70:1098–1103
Chinnam PR, Zhang H, Wunder SL (2015) Blends of pegylatedpolyoctahedralsilsesquioxanes (POSS-PEG) and methyl cellulose as solid polymer electrolytes for lithium batteries. Electrochim Acta 170:191–201
Kim JH, Won J, Kang YS (2004) Olefin-induced dissolution of silver salts physically dispersed in inert polymers and their application to olefin/paraffin separation. J Membr Sci 241:403–407
Borgohain MM, Joykumar T, Bhat SV (2010) Studies on a nanocomposite solid polymer electrolyte with hydrotalcite as a filler. Solid State Ion 181:964–970
Samsudin AS, Saadiah MA (2018) Ionic conduction study of enhanced amorphous solid bio-polymer electrolytes based carboxymethyl cellulose doped NH4Br. J Non Cryst Solids 497:19–29. https://doi.org/10.1016/j.jnoncrysol.2018.05.027
Mohd FH, Nur SNA (2019) Conductivity and transport properties of starch/glycerin-MgSO4 solid polymer electrolytes. Int J Adv Appl Sci 5:38–43
Yalinca Z, Mohammed DAK, Hadi JM, Yilmaz E (2016) Effect of CaCO3/HCl pretreatment on the surface modification of chitin gel beads via graft copolymerization of 2-hydroxy ethyl methacrylate and 4-vinylpyridine. Int J Biol Macromol 82:208–216. https://doi.org/10.1016/j.ijbiomac.2015.10.049
Ignjatovic N, Wu V, Ajdukovic Z, Mihajilov-Krstev T, Uskokovic V, Uskokovic D (2016) Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater Sci Eng C 60:357–364
Du BW, Hu SY, Singh R et al (2017) Eco-friendly and biodegradable biopolymer chitosan/Y2O3 composite materials in flexible organic thin-film transistors. Materials (Basel) 10. https://doi.org/10.3390/ma10091026
Rahman NA, Hanifah SA, Mobarak NN, Ahmad A, Ludin NA, Bella F et al (2021) Chitosan as a paradigm for biopolymer electrolytes in solid-state dye-sensitised solar cells. Polymer (Guildf) 230:124092. https://doi.org/10.1016/j.polymer.2021.124092
Wiedmann S, Kerscher B, Lienert C, Böcherer D, Mülhaupt R (2020) Tailoring poly(2-oxazoline)-based polymeric ionic liquids as thermoresponsive molecular brushes and programmable dispersants for silver nanoparticles. Macromolecules 53:6703–6710. https://doi.org/10.1021/acs.macromol.0c00267
Aziz SB, Karim WO, Brza MA, Abdulwahid RT, Saeed SR, Al-Zangana S, Kadir MFZ (2019) Ion transport study in CS: POZ based polymer membrane electrolytes using Trukhan model. Int J Mol Sci 20. https://doi.org/10.3390/ijms20215265
Lee WG, Kang SW (2016) Highly selective polymer electrolyte membranes consisting of poly(2-ethyl-2-oxazoline) and Cu(NO3)2 for SF6 separation. Sci Rep 6:2–7. https://doi.org/10.1038/srep20430
Schainker RB (2004) Executive overview: energy storage options for a sustainable energy future. Power Eng Soc Gen Meet 2:1–6
Muchakayala R, Song S, Wang J, Fan Y, Bengeppagari M, Chen J, Tan M (2018) Development and supercapacitor application of ionic liquid-incorporated gel polymer electrolyte films. J Ind Eng Chem 59:79–89. https://doi.org/10.1016/j.jiec.2017.10.009
Yadav N, Mishra K, Hashmi SA (2017) Optimization of porous polymer electrolyte for quasi-solid-state electrical double layer supercapacitor. Electrochim Acta 235:570–582. https://doi.org/10.1016/j.electacta.2017.03.101
Kamarudin KH, Hassan M, Isa MIN (2018) Lightweight and flexible solid-state EDLC based on optimized CMC-NH4NO3 solid bio-polymer electrolyte. ASM Sci J 11:29–36
Zhang Y, Feng H, Wu X, Wang L, Zhang A, Xia T, Dong H, Li X, Zhang L (2009) Progress of electrochemical capacitor electrode materials: a review. Int J Hydrogen Energy 34(11):4889–4899. https://doi.org/10.1016/j.ijhydene.2009.04.005
Liew CW, Ramesh S, Arof AK (2016) Enhanced capacitance of EDLCs (electrical double layer capacitors) based on ionic liquid added polymer electrolytes. Energy 109:546–556. https://doi.org/10.1016/j.energy.2016.05.019
Lewandowski A, Zajder M, Frackowiak E, Béguin F (2001) Supercapacitor based on activated carbon and polyethylene oxide-KOH-H2O polymer electrolyte. Electrochim Acta 46:2777–2780. https://doi.org/10.1016/S0013-4686(01)00496-0
Svensson AM, Valøen LO, Tunold R (2005) Modeling of the impedance response of porous metal hydride electrodes. Electrochim Acta 50:2647–2653. https://doi.org/10.1016/j.electacta.2004.11.035
Parameswaran V, Nallamuthu N, Devendran P, Nagarajan ER, Manikandan A (2017) Electrical conductivity studies on ammonium bromide incorporated with zwitterionic polymer blend electrolyte for battery application. Phys B Condens Matter 515:89–98. https://doi.org/10.1016/j.physb.2017.03.043
Jacob MME, Prabaharan SRS, Radhakrishna S (1997) Effect of PEO addition on the electrolytic and thermal properties of PVDF-LiClO4 polymer electrolytes. Solid State Ionics 104:267–276. https://doi.org/10.1016/s0167-2738(97)00422-0
Fonseca CP, Cavalcante F Jr, Amaral FA, Souza CAZ, Neves S (2007) Int J Electrochem Sci 2:52–63
Pradhan DK, Choudhary P, Samantaray BK, Karan NK, Katiyar RS (2007) Effect of plasticizer on structural and electrical properties of polymer nanocomposite electrolytes. Int J Electrochem Sci 2:861–71
Winie T, Arof AK, Thomas S (2020) Polymer electrolytes characterization techniques and energy applications. Wiley-VCH, Weinheim, Germany
Scisco GP, Orazem ME, Ziegler KJ, Jones KS (2021) On the rate capability of supercapacitors characterized by a constant-phase element. J Power Sources 516:230700. https://doi.org/10.1016/j.jpowsour.2021.230700
Rajendran S, Babu R, shanker, Sivakumar P, (2008) Investigations on PVC/PAN composite polymer electrolytes. J Memb Sci 315:67–73. https://doi.org/10.1016/j.memsci.2008.02.007
Nofal MM, Hadi JM, Aziz SB, Brza MA, Asnawi ASFM, Dannoun EMA, Abdullah AM, Kadir MFZ (2021) A Study of methylcellulose based polymer electrolyte impregnated with potassium ion conducting carrier: impedance, EEC Modeling, FTIR, Dielectric, and Device Characteristics. Materials 14:4859. https://doi.org/10.3390/ma14174859
Mohapatra SR, Thakur AK, Choudhary RNP (2009) Effect of nanoscopic confinement on improvement in ion conduction and stability properties of an intercalated polymer nanocomposite electrolyte for energy storage applications. J Power Sources 191:601–613. https://doi.org/10.1016/j.jpowsour.2009.01.100
Abdullah AM, Aziz SB, Saeed SR (2021) Structural and electrical properties of polyvinyl alcohol (PVA):methyl cellulose (MC) based solid polymer blend electrolytes inserted with sodium iodide (NaI) salt. Arab J Chem 14:103388. https://doi.org/10.1016/j.arabjc.2021.103388
Fuzlin AF, Mazuki NF, Khan NM, Saadiah MA, Hasan MM, Nagao Y et al (2022) Studies on the ions transportation behavior of alginate doped with H+ carrier-based polymer electrolytes. Mater Chem Phys 287:126207. https://doi.org/10.1016/j.matchemphys.2022.126207
Teo LP, Buraidah MH, Nor AFM, Majid SR (2012) Conductivity and dielectric studies of Li2SnO3. Ionics (Kiel) 18:655–665. https://doi.org/10.1007/s11581-012-0667-2
Khutoryanskaya OV, Khutoryanskiy VV, Pethrick RA (2005) Characterisation of blends based on hydroxyethylcellulose and maleic acid-alt-methyl vinyl ether. Macromol Chem Phys 206(15):1497–1510
Pradhan DK, Choudhary RNP, Samantaray BK (2008) Studies of structural, thermal and electrical behavior of polymer nanocomposite electrolytes. Express Polym Lett 2(9):630–638
Shukur MF, Hamsan MH, Kadir MFZ (2019) Investigation of plasticized ionic conductor based on chitosan and ammonium bromide for EDLC application. Mater Today Proc 17:490–498. https://doi.org/10.1016/j.matpr.2019.06.490
Chudpooti N, Doychinov V, Hong B, Akkaraekthalin P, Robertson I, Somjit N (2018) Multi-modal millimeter-wave sensors for plastic polymer material characterization. J Phys D Appl Phys 51(27):275103
Woo HJ, Majid SR, Arof AK (2012) Dielectric properties and morphology of polymer electrolyte based on poly(ε-caprolactone) and ammonium thiocyanate. Mater Chem Phys 134:755–761. https://doi.org/10.1016/j.matchemphys.2012.03.064
Chisca S, Musteata VE, Sava I, Bruma M (2011) Dielectric behavior of some aromatic polyimide films. Eur Polym J 47:1186–1197. https://doi.org/10.1016/j.eurpolymj.2011.01.008
Kurien S, Mathew J, Sebastian S et al (2006) Dielectric behavior and ac electrical conductivity of nanocrystalline nickel aluminate. Mater Chem Phys 98:470–476. https://doi.org/10.1016/j.matchemphys.2005.08.080
Matsuda Y, Inoue K, Takeuchi H, Okuhama Y (1998) Gel polymer electrolytes for electric double layer capacitors. Solid State Ionics 113:103–107
Sangeetha M, Mallikarjun A, Aparna Y, Reddy MV, Kumar JS, Sreekanth T et al (2021) Dielectric studies and AC conductivity of PVDF-HFP: LiBF4: EC plasticized polymer electrolytes. Mater Today Proc 44:2168–2172. https://doi.org/10.1016/j.matpr.2020.12.280
Rani MSA, Ahmad A, Mohamed NS (2018) A comprehensive investigation on electrical characterization and ionic transport properties of cellulose derivative from kenaf fibre-based biopolymer electrolytes. Polym Bull 75:5061–5074
Jayathilaka PARD, Dissanayake MAKL, Albinsson I, Mellander B-E, (2003) Dielectric relaxation, ionic conductivity and thermal studies of the gel polymer electrolyte system PAN/EC/PC/LiTFSI. Solid State Ion 156:179–195
Pawlicka A, Tavares FC, Dörr DS, Cholant CM, Ely F, Santos MJL et al (2019) Dielectric behavior and FTIR studies of xanthan gum-based solid polymer electrolytes. Electrochim Acta 305:232–239. https://doi.org/10.1016/j.electacta.2019.03.055
Khatri P, Behera B, Srinivas V, Choudhary RNP (2009) Structural and dielectric properties of Ba3V2O8 ceramics. Curr Appl Phys 9(2):515–519
Anandha Jothi M, Vanitha D, Nallamuthu N, Manikandan A, Asath BS (2020) Investigations of lithium ion conducting polymer blend electrolytes using biodegradable cornstarch and PVP. Phys B Condens Matter 580:411940. https://doi.org/10.1016/j.physb.2019.411940
Smaoui H, Mir LEL, Guermazi H, Agnel S, Toureille A (2009) Study of dielectric relaxations in zinc oxide-epoxy resin nanocomposites. J Alloys Compd 477:316–321. https://doi.org/10.1016/j.jallcom.2008.10.084
Hamsan MH, Aziz SB, Kadir MFZ et al (2020) The study of EDLC device fabricated from plasticized magnesium ion conducting chitosan based polymer electrolyte. Polym Test 90:106714. https://doi.org/10.1016/j.polymertesting.2020.106714
Morsi MA, Oraby AH, Elshahawy AG, Abd El-Hady RM (2019) Preparation, structural analysis, morphological investigation and electrical properties of gold nanoparticles filled polyvinyl alcohol/carboxymethyl cellulose blend. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2019.09.07
Hadi JM, Aziz SB, Ghafur Rauf H, Abdulwahid RT, Al-Saeedi SI, Tahir DA, Kadir MFZ (2022) Proton conducting polymer blend electrolytes based on MC: FTIR, ion transport and electrochemical studies. Arab J Chem 15:104172. https://doi.org/10.1016/j.arabjc.2022.104172
Castillo J, Chacón M, Castillo R, Vargas RA, Bueno PR, Varela JA (2009) Dielectric relaxation and dc conductivity on the PVOH-CF3COONH4 polymer system. Ionics (Kiel) 15:537–544. https://doi.org/10.1007/s11581-009-0320-x
Dave G, Kanchan DK (2018) Dielectric relaxation and modulus studies of PEO-PAM blend based sodium salt electrolyte system. Indian J Pure Appl Phys 56:978–988
Brza MA, Aziz SB, Anuar H, Dannoun EMA, Ali F, Abdulwahid RT et al (2020) The study of EDLC device with high electrochemical performance fabricated from proton ion conducting PVA-based polymer composite electrolytes plasticized with glycerol. Polymers (Basel) 12:1896. https://doi.org/10.3390/POLYM12091896
Dhatarwal P, Sengwa RJ (2020) Dielectric relaxation, Li-ion transport, electrochemical, and structural behaviour of PEO/PVDF/LiClO4/TiO2/PC-based plasticized nanocomposite solid polymer electrolyte films. Compos Commun 17:182–191. https://doi.org/10.1016/j.coco.2019.12.006
Aziz SB (2016) Occurrence of electrical percolation threshold and observation of phase transition in chitosan (1–x): AgI x (0.05≤ x≤ 0.2)-based ion-conducting solid polymer composites. Appl Phys A 122:1–13
Yusof YM et al (2014) The effect of plasticization on conductivity and other properties of starch/chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Mol Cryst Liq Cryst 603(1):73–88
Li Wangyu et al (2017) A PEO-based gel polymer electrolyte for lithium ion batteries. RSC Adv 7(38):23494–23501
Kadir Mohd Fakhrul, Zamani, et al (2018) Biopolymeric electrolyte based on glycerolized methyl cellulose with NH4Br as proton source and potential application in EDLC. Ionics 246:1651–1662
Rani ASmal Mohd Saiful et al (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 6(9):2371–2385
Shukur MF, Hamsan MH, Kadir MFZ (2019) Investigation of plasticized ionic conductor based on chitosan and ammonium bromide for EDLC application. Mater Today Proc 17:490–498. https://doi.org/10.1016/j.matpr.2019.06.490
Kadir MFZ, Arof AK (2013) Application of PVA–chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Mater Res Innov 1(5):217–220
Pérez-Madrigal MM, Edo MG, Alemán C (2016) Powering the future: application of cellulose-based materials for supercapacitors. Green Chem 18:5930–5956. https://doi.org/10.1039/c6gc02086k
Liew CW, Ramesh S (2015) Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr Polym 124:222–228
Hadi JM, Aziz SB, Brza MA, Kadir MFZ, Abdulwahid RT, Ali Al-Asbahi B, Ahmed Ali Ahmed A (2020) Structural and energy storage behavior of ion conducting biopolymer blend electrolytes based on methylcellulose: dextran polymers. Alexandria Eng. J. 61:9273–9285. https://doi.org/10.1016/j.aej.2022.03.042
Shuhaimi NEA, Teo LP, Woo HJ et al (2012) Electrical double-layer capacitors with plasticized polymer electrolyte based on methyl cellulose. Polym Bull 69:807–826. https://doi.org/10.1007/s00289-012-0763-5
Hamsan MH, Shukur MF, Kadir MFZ (2017) NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics 23:3429–3453
Maheshwari T, Tamilarasan K, Selvasekarapandian S, Chitra R, Kiruthika S (2021) Investigation of blend biopolymer electrolytes based on dextran-PVA with ammonium thiocyanate. J Solid State Electrochem 25(3):755–765. https://doi.org/10.1007/s10008-020-04850-5
Hamdan KZ, Khiar ASA (2014) Conductivity and dielectric studies of methylcellulose/chitosan-NH 4CF3SO3 polymer electrolyte. Key Eng Mater 594–595:818–822. https://doi.org/10.4028/www.scientific.net/KEM.594-595.818
Shetty SK, Ismayil SG (2020) Enhancement of electrical and optical properties of sodium bromide doped carboxymethyl cellulose biopolymer electrolyte films. J Macromol Sci Part B Phys 59:235–247. https://doi.org/10.1080/00222348.2020.1711585
Agrawal SL, Singh M, Tripathi M, Dwivedi MM, Pandey K (2009) Dielectric relaxation studies on [PEO-SiO2]:NH4SCN nanocomposite polymer electrolyte films. J Mater Sci 44:6060–6068. https://doi.org/10.1007/s10853-009-3833-9
Funding
This research was supported by the Ministry of Higher Education (MoHE) of Malaysia through Fundamental Research Grant Scheme (FRGS/1/2019/STG07/UM/02/6). The authors also gratefully acknowledge the financial support for this study from the Ministry of Higher Education and Scientific Research-Kurdish National Research Council (KNRC), Kurdistan Regional Government/Iraq. The financial support from the University of Sulaimani is greatly appreciated.
Author information
Authors and Affiliations
Contributions
Jihad M Hadi; writing original draft, data curation, methodology, Shujahadeen Aziz; conceptualization and methodology, Rebar T Abdulwahid; methodology, validation, editing and review, Mohamad A Brza, Hawahin Tahir, Samir M Hamad, H.J. Woo, Y. Alias, M.H. Hamsan; review and conceptualization; N.A. Shamsuri; editing and review; O J S Steve; industrial linkage, methodology, editing and review, M.F.Z. Kadir; supervision, writing, editing, review, methodology, and funding.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hadi, J.M., Aziz, S.B., Abdulwahid, R.T. et al. Characterizing sodium-conducting biopolymer blend electrolytes with glycerol plasticizer for EDLC application. Ionics 30, 2409–2423 (2024). https://doi.org/10.1007/s11581-024-05412-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05412-9