Abstract
Antibiotic nanocomposite polymers show great promise in treating a variety of pathogens that cause widespread disease. Sodium alginate-grafted diphenylamine (NaAlg-g-DPA) embedded with different ratios of silver nanoparticles (AgNPs) was fabricated and characterized through different techniques including FTIR, XRD, and SEM techniques for investigating the antimicrobial activity. XRD confirmed the crystallinity of these compounds, and the average crystal size of Na Alg-g-DPA/Ag was estimated to be 48.6 nm. Then it was applied as an antimicrobial agent and evaluated through two ways (inhibition zone and MIC techniques) against Staphylococcus aureus as gram-positive bacteria with an inhibition zone of 19.31.6 mm and 18.60.63 mm against Escherichia coli as gram-negative bacteria while with increasing the Ag ratio 2:1 there was an enhancement in their biological activity to be 21.90.69 mm against Staphylococcus aureus and with an inhibition zone of 21.32.1 mm against Escherichia coli. The outcomes of this investigation are important for the development of new composite materials with antibacterial properties for industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most significant issues that cause a threat to millions of people worldwide is microbial infection. Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enteritidis, and Aspergillus niger are the most pathogenic microorganisms that cause many diseases, including nausea, pneumonia, abdominal pain, enterocolitis, pyogenic liver abscess, hemorrhagic colitis, and diarrhea [1,2,3,4,5,6].
Consequently, many efforts were made to control this problem through developing new effective antimicrobial systems [7]. The development of antimicrobial resistance is considered the main target for antimicrobial drug delivery research. Biopolymer antimicrobials are the most widely used materials owing to their cost-effectiveness and ease of modification [8, 9].
Sodium alginate (biopolymer) is a group of naturally occurring anionic polysaccharides derived from brown seaweed and is generally considered biocompatible, non-immunogenic, and non-toxic polymers [10]. Alginates constitute a huge portion of the earth’s organic matter because they are synthesized by living organisms such as plants, animals, bacteria, and fungi during their entire life cycle [11, 12]. Alginate has been grafted with different engineered polymers such as polyacrylamide (PAAm), polypyrrole (Ppy), and polydiphenylamineaniline (PDPA) to optimize the restriction of these synthetic polymers and improve their biodegradability and eco-friendly nature. The grafting of the manufactured polymer onto the biopolymer will improve the antimicrobial action against diverse species of organisms [13,14,15,16].
Polydiphenylamine (PDPA) is a significant polymer used as an antimicrobial agent for a long time due to the presence of nitrogen between two rings [17]. Diphenylamine derivatives have been shown to have a variety of biological activities, including anthelmintic activity (e.g., amoscanate [18]), analgesic and anti-inflammatory activity (e.g., diclofenac, tolfenamic acid [19]), and anticonvulsant activity (e.g., retigabine [20]).
In addition to the incorporation of different nanoparticles on polymer composite matrix are active functional hybrid materials containing nanoparticle and a polymer matrix. These materials have a wide range of beneficial uses in antimicrobial activity. The strengthened synergistic interaction between the constituent parts and the conductive network within the polymer matrix enhanced their activity.
Generally, one of the most popular materials for providing a variety of substrates with exceptional biological and mechanical properties is grafted polymer containing silver nanoparticles (AgNPs), which is also capable of resolving the common instability problem associated with the use of AgNPs-based coatings for fabrics after washing [21,22,23,24,25,26].
However, silver nanoparticles (Ag NPs) are most widely used in the evolution of new broad spectrum antimicrobial agents against pathogenic microorganisms. Silver nanoparticles are considered as potential therapeutic agents with a significant impact on respiratory medicine, via offering a multifunctional platform that could treat bacterial infections. They are synthesized by means of chemical reduction using highly reactive reducing agents [27, 28].
AgNPs have piqued the interest of researchers due to their electronic properties, appealing optical properties, and excellent antimicrobial activities [29,30,31]. AgNPs have strong cytotoxicity for a wide range of microorganisms and are widely used as an antibacterial agent [32, 33].
Hence, the introduction of diphenylamine into sodium alginate also silver nanoparticles were embedded into the grafting substances to produce a new effective, safe, and cheap antimicrobial agent against various bacteria and fungi by a combination (Na.alg-g-DPA) with silver nano-particles with different concentrations of AgNPs of 1:1 and 1:2 AgNPs/Na.Alg-g-DPA ratio (Fig. 1). The new materials were characterized by FTIR, X-ray diffraction, and scanning and transmission electron microscopes. The new materials were tested against gram-positive strains (Streptococcus pneumoniae ATCC 6303 and Staphylococcus aureus ATCC 29213), gram-negative strains (Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922), and fungi (Aspergillus fumigatus ATCC 13073 and Candida albicans ATCC 10231).
Materials and methods
Materials
Silver nitrate [TECHNO PHARMCHEM], glucose [Bio-chemical], polyvinyl pyrrolidine, sodium alginate, and ammonium persulfate were purchased from Sigma-Aldrich. Diphenylamine was purchased from Oxford Lab Chem. Hydrochloric acid and ethyl alcohol were purchased from Piochem.
Methods
Synthesis of NaAlg-g-DPA
In flask (1), 25 ml of distilled water, 0.54 gm of sodium alginate (NaAlg), 2.1 gm of diphenylamine (DPA), and 1.9 ml of 1 M HCl were placed and stirred at 500 rpm for 1 h. In the flask (2), put 1 M of HCl (1.9 ml) and 0.5 M of Amm. per sulfite (APS) (2.85 gm) as an initiator in 25 ml of distilled water that was stirred at 500 rpm for 1 h. Flask (1) was slowly and carefully added to flask (2) under continuous stirring for 2 h. The system was kept for 24 h at room temperature. The solid product was separated by filtration and extracted by Soxhlet with distilled water and ethyl alcohol. The obtained solid was then dried for 24 h at 40 °C [34]. Soxhlet extraction is a method for controlling polymer solubility via a post-synthesis step. It is utilized for the extraction of a polymer from the impurities using a solvent in which the polymer is soluble.
Preparation of silver nanoparticles
At three molar ratios, Ag:PVP is 1:1, 1:1.5, and 1:2. After dissolving 3.4 gm of silver nitrate (AgNO3) in 20 ml of H2O in beaker (A) and 10.2 gm of polyvinyl pyrrolidone (PVP) at a 1:1 ratio, 0.24 gm of NaOH, and 1.08 gm of glucose in 60 ml of H2O in beaker (B), the mixture was stirred continuously at 600 C, and the product was centrifuged several times. By repeating the steps for other ratios of Ag: PVP 1:1.5, 1:2 at the weight of PVP 15.3 gm, 20.4 gm, and Ag 3.4 gm.
PVP is crucial to the creation of silver NPs. It stops the created AgNPs from aggregating. The process through which PVP in an aqueous solution reduces silver ions to nanoparticles. Initially, a coordinative complex is created between silver ions and PVP by the donation of loan pair electrons from PVP's oxygen and nitrogen atoms to the silver's Sp orbitals. PVP encourages the nucleation of the metallic silver reduced by glucose in the second stage. Finally, PVP's steric action prevents the formation of silver grains and particles.
Preparation of sodium alginate-poly-diphenylamine silver nanoparticles (Fig. 2)
NaAlg-g-DPA/AgNPS weight ratios of 1:1 and 2:1 In Beaker (A), we put diphenylamine (DPA), synthesized AgNPs, Na-alginate, and HCL at 0.532 gm, 1.005 gm, 1.262 gm, and 0.87 ml, respectively, in 12 ml of H2O, stirring for 1 h. In the beaker (B), put 0.87 ml of HCL and 1.166 gm of Amm. Persulfate (APS) and stirring for 1 h, then a beaker (B) was added to (A) dropwise. Finally, the mixture was stirred for 2 h. Finally, filter the product, wash it, and dry it very well.
Instrumentations
Infrared spectroscopy
To confirm the occurrence of the reaction, using a Fourier transform infrared (FTIR) spectrometer (VERTEX 70 FT-IR). FT-IR spectra were recorded in ATR discs at room temperature within the wavenumber range of 4000–600 cm−1.
X-ray diffraction
The crystalline structure of prepared copolymers was measured by X-ray diffraction (2020964 PA Analytical Empyrean), which is a technique that uniquely provides phase identification (e.g. graphite or diamond), along with phase crystallite size and quantification.
Scanning electron microscope
By using a scanning electron microscope (JEOL (JSM-5200)), the morphologies of the prepared copolymers were measured. Samples that prepared by placing a slight part of a film on a carbon tube on a stub, which was coated with a gold thin layer.
Results and discussion
Characterization
FTIR spectroscopy
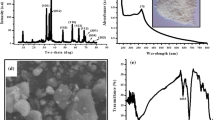
Figure 3 shows the FTIR spectroscopy of AgNPs, Na.Alg-g-DPA and Na.Alg-g-DPA: AgNPs 2:1, respectively. All of the characteristic peaks for AgNPs/PVP are at 2923, 1460, and 1374 cm−1, respectively. The peak at 1285–1295 cm−1, typical for the C–N bond in PVP, was also presented. In AgNPs/PVP, a strong absorption peak, which is characteristic of the carbonyl group of PVP at 1650–1660 cm−1, was observed. The peak was typical for the C–N bond at 1295 cm−1. These peaks were associated with the formation of coordination bonds between silver atoms and oxygen or nitrogen atoms arising from PVP units [35]. In the Na.alg-g-DPA diagram in Fig. 3, the absorption peak at 3382 cm−1 corresponds to the O–H group, whose variations are shaped because of the contribution toward the reduction and stabilization processes. Other peaks appear at 1746, 1594, and 1501 cm−1, which were attributed to the stretching vibration of the benzidine structure C = C and/or in C = N in the suggested polymer structure, while there are three strong absorption bands that appear at 745, 809, and 875 cm−1, which were ascribed to out of plane C–H deformation showing 1,4-disubstitution in the benzene ring [36]. After incorporation of AgNPs into the grafting process, the peaks of 1501 and 1313 cm−1 shifted to 1485 and 1311 cm−1, and 1029 cm−1, indicating metal oxide nanomaterials, the hydroxyl groups, and carboxyl groups are shifted from 3382 and 1746 cm−1 to 3385 and 1741 cm−1. The variations of the hydroxyl and carboxylate groups have been reported in the previous study on the synthesis of AgNPs with another polysaccharide [37, 38].
XRD spectroscopy
Figure 4A–C shows the XRD patterns of AgNPs, NaAlg-g-DPA, and NaAlg-g-DPA/Ag, respectively. In the case of AgNPs (Fig. 4A), there are main peaks at 20.36°, 28.04°, 32.4°, and 38.3°, which appear in many works in which the XRD pattern includes the relevant 2 range. These peaks are due to the crystalline and amorphous organic phases accompanying crystallized AgNPs [39, 40]. The grafted sample (Fig. 4B) showed new diffraction peaks at 2 = 9.6°, 19.5°, 21°, and 28°, which related to DPA [41] that helped us in confirming the grafting process [42, 43]. As shown in Fig. 3C, the main peak corresponding to 100% appeared at 38.5°, while other peaks shifted to 47°, 65°, and 78°, indicating that the Ag NPs were spherical in structure and crystalline in nature [44]. The sharp peaks almost indicated some bioorganic compounds or proteins in the NPS during synthesis [45]. These results prove the good incorporation of grafted polymer with Ag NPs. The average crystallite size (D) of Na.alg-g-DPA/Ag was calculated according to the Scherrer equation to be 48.6 nm.
Scanning electron microscopes
The scanning electron microscope (SEM) of NaAlg-g-DPA and NaAlg-g-DPA/Ag is shown in Fig. 5A–C. It was observed that sodium alginate's surface has been covered with a poly(DPA) grafted chain (Fig. 5A). A porous surface is produced by the ribose structure of grafted PDPA polymer on the sodium alginate surface. This novel surface structure is anticipated to enhance the antimicrobial activity of grafted polymer. After incorporation of AgNPs into the grafted polymer matrix, Fig. 5B, C shows small aggregates from AgNPs appeared on the surface of grafted Na.Alg-g-DPA. Additionally, the crystal size of the graft (Na.Alg-g-DPA) and AgNPs was 54.59 nm and 0.2 nm, respectively. The particle size was greater than the crystallite size obtained from XRD. This suggests that the apparent SEM particles may have consisted of more than one XRD crystallite. The AgNPs particles have a spherical shape stacked in regular shape, which proved the incorporation of AgNPs. The homogenous porous structure of NaAlg-g-DPA/Ag improves the antimicrobial activity of this compound [40].
Characterization by 1H NMR
The 1HNMR spectra of alginate and NaAlg-g-DPA are shown in Fig. 6. It was found that the backbone of alginate shows in obvious peaks at 4.0–4.5 ppm (Fig. 6a). In addition to NaAlg peaks, a new peaks appeared related to DPA δ7.25 (t, J = 7.0 Hz, 4H), 7.07 (d, J = 7.7 Hz, 4 H), 6.92 (t, J = 7.3 Hz, 2H) which confirm the successful grafting of DPA onto NaAlg successfully [33].
Antimicrobial activity
Nanotechnology's uses in the biomedical field have grown in recent years, with atomic-scale functional materials being used to fight and inhibit diseases. Two techniques (inhibition zone technique and minimum inhibitory concentration (MIC)) were used for antimicrobial evaluation of sodium alginate and the newly synthesized compounds (1–4). Different species of microbes were used for these tests, including Streptococcus pneumoniae (ATCC 6303), Staphylococcus aureus (ATCC 29213), as gram-positive bacteria, Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922) as gram-negative bacteria, and the antifungal activity against Aspergillus fumigatus (ATCC 13073), and Candida albicans (ATCC 10231). Tables 1 and 2 illustrate the inhibition zone diameter of the tested compounds against gram-positive and gram-negative bacteria and fungi.
Concerning sodium alginate (compound1), it has the lowest antimicrobial activity compared to the other synthesized compounds listed in Table 1, whereas the activity of grafted polymer was improved due to the presence of DPA chains, which may interact with the bacterial membrane via an electrostatic mechanism that causes a change in the potential gradient, which disrupts the cells [46].
However, the incorporation of AgNPs in grafted material (NaAlg-g-DPA/Ag) will enhance the antimicrobial activity and increased linearly with the ratios of AgNPs in the nanocomposites. AgNPs nanoparticles are potent candidates as antimicrobials owing to their intrinsic properties and excellent thermal stability. These particles release silver ions which interact with bacterial cell walls causing breakage of the cell membrane [47].
The microbial activity significantly increased with the presence of AgNPs (compound3 with 1:1). Its inhibition zone was 18.50.58 and 19.31.6 mm against Streptococcus pneumoniae and Staphylococcus aureus as gram-positive bacteria, 17.41.2 and 18.60.63 mm against Pseudomonas aeruginosa and Escherichia coli as gram-negative bacteria, and 15.41.3 and 18.22.1 mm against Aspergillus fumigatus and Candida albicans as fungi, respectively.
The biological activity increases with increasing AgNPs ratios of 2: 1 (compound 4) to (20.60.85 and 21.90.69 mm against Streptococcus pneumoniae and Staphylococcus aureus as gram-positive bacteria, 19.41.4 and 21.32.1 mm against Pseudomonas aeruginosa and Escherichia coli as gram-negative bacteria, and 20.71.4 and 21.82.1 mm against Aspergillus fum.
MIC is considered to be the significant standard technique to evaluate the susceptibility of a microorganism to a particular antibiotic and is performed as reported earlier [48, 49]. Table 2 represents the MIC values of the tested compounds (1–4) against the aforementioned organisms. These values are considered another evidence of the biological activity of the tested compounds, which also showed that NaAlg-g-DPA/Ag with a 2:1 ratio showed higher antibacterial and antifungal activity than others.
Conclusion
In this study, we were interested in new polymers containing different ratios of Ag NPs. The development of the novel class of composites depends crucially on their ability to resist different types of microbes. A new antimicrobial agent has been synthesized by grafting sodium alginate with diphenylamine and the incorporation of silver nanoparticles for evaluation against different types of bacteria and fungi using the agar diffusion method. The effect of the silver nanoparticle dopant ratio of the composite was studied in terms of antimicrobial activities. Pure grafted polymer showed a good antimicrobial effect with MIC 62.5, and increasing the content of Ag NPs doping significantly enhanced its performance against (gram-negative, gram-positive, and fungi) with MIC 3.9 and 0.98 for 1:1 and 2:1, respectively. These findings suggest that the DPA modified with Ag NPs has excellent scope for further development as a commercial antimicrobial agent. Further experiments were needed to elucidate their mechanism of action.
References
Krishnaraj C, Ramachandran R, Mohan K, Kalaichelvan PT (2012) Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim Acta - Part A Mol Biomol Spectrosc 93:95–99. https://doi.org/10.1016/j.saa.2012.03.002
Wang H, Wang N, Wang B, Zhao Q, Fang H, Fu C et al (2016) Antibiotics in drinking water in Shanghai and their contribution to antibiotic exposure of school children. Environ Sci Technol 50:2692–2699. https://doi.org/10.1021/acs.est.5b05749
Wang G, Zhou S, Han X, Zhang L, Ding S, Li Y et al (2020) Occurrence, distribution, and source track of antibiotics and antibiotic resistance genes in the main rivers of Chongqing city, southwest China. J Hazard Mater 389:122110. https://doi.org/10.1016/j.jhazmat.2020.122110
Li F, Chen L, Chen W, Bao Y, Zheng Y, Huang B et al (2020) Antibiotics in coastal water and sediments of the East China Sea: distribution, ecological risk assessment and indicators screening. Mar Pollut Bull 151:110810. https://doi.org/10.1016/j.marpolbul.2019.110810
Elella MA, Abdel-Aziz* MM, Abd El-Ghany NA (2021) Synthesis of a high-performance antimicrobial o-quaternized alginate: a promising potential antimicrobial agent. Cellul Chem Technol 55(1–2):75–86
Abu-Thabit NY, Uwaezuoke OJ (2022) Superhydrophobic nanohybrid sponges for separation of oil/ water mixtures. Chemosphere 294:133644
Langade CU (2019) Evaluation of antimicrobial activity of holopteliya integrifolia. Int J Sci Res 8:786–789
Goda ES, Elella MHA, Sohail M, Singu BS, Pandit B, El Shafey AM, Aboraia AM, Gamal H, Hong SE, Yoon KR (2021) N-methylene phosphonic acid chitosan/graphene sheets decorated with silver nanoparticles as green antimicrobial agents. Int J Biol Macromol 182:680–688
Pandit B, Goda ES, Abu Elella MH, ur Rehman A, Hong SE, Rondiya SR, Barkataki P, Shaikh SF, Al-Enizi AM, El-Bahy SM, Yoon KR (2022) One-pot hydrothermal preparation of hierarchical manganese oxide nanorods for high-performance symmetric supercapacitors. J Energy Chem 65:116–126
Sachan N, Pushkar S, Jha A, Bhattcharya A (2009) Sodium alginate: the wonder polymer for controlled drug delivery. J Pharm Res 2:1191–1199
Ngwuluka NC, Abu-Thabit NY, Uwaezuoke OJ, Erebor JO, Ilomuanya MO, Mohamed RR, Soliman SMA, Elella MHA, Ebrahim NAA (2021) Natural polymers in micro- and nanoencapsulation for therapeutic and diagnostic applications: Part II—polysaccharides and proteins. Nano- Microencapsul. https://doi.org/10.5772/intechopen.95402
Abu Elella MH, Hanna DH, Mohamed RR, Sabaa MW (2021) Synthesis of xanthan gum/trimethyl chitosan interpolyelectrolyte complex as pH-sensitive protein carrier. Polym Bull. https://doi.org/10.1007/s00289-021-03656-3
Abu Elella MH, Goda ES, Gab-Allah MA, Hong SE, Lijalem YG, Yoon KR (2022) Biodegradable polymeric nanocomposites for wastewater treatment. In: Advances in nanocomposite materials for environmental and energy harvesting applications, pp 245–298. doi:https://doi.org/10.1007/978-3-030-94319-6_9
Abu Elella MH, Goda ES, Gab-Allah MA, Hong SE, Pandit B, Lee S, Gamal H, Rehman A, Yoon KR (2020) Xanthan gum-derived materials for applications in environment and eco-friendly materials: a review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.104702
Abu-Elella MH, Goda* ES, Yoon KR, Hong SE, Morsy MS, Sadak RA, Gamal H (2021) Novel vapor polymerization for integrating flame retardant textile with multifunctional properties. Compos Commun 24: 100614
Abu Elella MH (2021) Synthesis and potential applications of modified Xanthan Gum. J Chem Eng Res Updates. https://doi.org/10.15377/2409-983X.2021.08.6
Kumar A, Mishra A (2015) Synthesis and antimicrobial activity of some new diphenylamine derivatives. J Pharm Bioallied Sci 7:81–85. https://doi.org/10.4103/0975-7406.148774
Franz M, Zahner H, Mehlhorn H, Striebel P (1990) Parasitnlngy 21833:393–400
Li Y, Qi X, Ming XX, Wu XF, Wu YF, Chen M et al (2009) The relationship between diphenylamine structure and NSAIDs-induced hepatocytes injury. Toxicol Lett 186:111–114. https://doi.org/10.1016/j.toxlet.2009.01.005
Douša M, Srbek J, Rádl S, Černý J, Klecán O, Havlíček J et al (2014) Identification, characterization, synthesis and HPLC quantification of new process-related impurities and degradation products in retigabine. J Pharm Biomed Anal 94:71–76. https://doi.org/10.1016/j.jpba.2014.01.042
Goda ES, Eissa AA-S, Pandit B, Abu-Elella MH (2022) Chalcogenides and phosphides for high-performance supercapacitors. Nanostruct Mater Supercapacitors. https://doi.org/10.1007/978-3-030-99302-3_19
Goda ES, Abu-Elella MH, Hong SE, Pandit B, Yoon KR (2021) Heba Gamal, smart flame retardant coating containing carboxymethyl chitosan nanoparticles decorated graphene for obtaining multifunctional textiles. Cellulose 28:5087–5105. https://doi.org/10.1007/s10570-021-03833-7
Abu-Elella MH, Goda ES, Abdallah HM, Elyamny S, Gamal H, Abu-Serea ES, Shalan AE (2022) Electroactive polymers for electrochromic applications. Electroactive Polymeric Materials, 1st Edition, (39)
Acik G, Altinkok C, Olmez H, Tasdelen MA (2018) Antibacterial film from chlorinated polypropylene via CuAAC click chemistry. Progr Organ Coat 125:73–78
Kalali EN, Guo W, Wang X, Xing W, Song L, Hu Y (2019) Effect of metal-based nanoparticles decorated graphene hybrids on flammability of epoxy nanocomposites. Compos: Part A 129:105694. https://doi.org/10.1016/j.compositesa.2019.105694
Abu-Elella MH, Sabaa MW, Hanna DH, Abdel-Aziz MM, Mohamed RR (2020) Antimicrobial pH-sensitive protein carrier based on modified xanthan gum. J Drug Deliv Sci Technol. https://doi.org/10.1016/j.jddst.2020.101673
Abdel-Aziz MM, Abu-Elella MH, Mohamed RR (2020) Green synthesis of quaternized chitosan/silver nanocomposites for targeting mycobacterium tuberculosis and lung carcinoma cells (A-549). Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.09.096
Abu-Elella MH, Goda ES, Abdallah HM, Shalan AE, Gamal H, Yoon KR (2021) Innovative bactericidal adsorbents containing modified xanthan gum/montmorillonite nanocomposites for wastewater treatment. Int J Biol Macromol 167:1113–1125. https://doi.org/10.1016/j.ijbiomac.2020.11.065
Wang H, Qiao X, Chen J, Ding S (2005) Preparation of silver nanoparticles by chemical reduction method. Colloids Surf A Physicochem Eng Asp 256:111–115. https://doi.org/10.1016/j.colsurfa.2004.12.058
Li D, He Q, Cui Y, Li J (2014) F of pH-responsive nanocomposites of gold, Nanoparticles/poly(4-vinylpyridine) CM 19 (2007) 412–417. Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf A Physicochem Eng Asp 444:180–188. https://doi.org/10.1016/j.colsurfa.2013.12.008
Jyothi A, Sashidhar RB, Arunachalam J (2010) Gum kondagogu ( Cochlospermum gossypium): a template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydr Polym 82:670–679. https://doi.org/10.1016/j.carbpol.2010.05.034
Wei D, Qian W (2008) Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf B: Biointerfaces 62:136–142. https://doi.org/10.1016/j.colsurfb.2007.09.030
Ahmed A, Mohamed F, Elzanaty AM, Abdel-Gawad OF (2021) Synthesis and characterization of diphenylamine grafted onto sodium alginate for metal removal. Int J Biol Macromol 176:766–776. https://doi.org/10.1016/j.ijbiomac.2020.11.159
Ncube S, Lekoto G, Cukrowska E, Chimuka L (2018) Development and optimisation of a novel three-way extraction technique based on a combination of Soxhlet extraction, membrane assisted solvent extraction and a molecularly imprinted polymer using sludge polycyclic aromatic hydrocarbons as model compounds. J Sep Sci 41:918–928
Mohamed F, Bhnsawy N, Shaban M (2021) Reusability and stability of a novel ternary (Co–Cd–Fe)-LDH/PbI2 photoelectrocatalytst for solar hydrogen production. Sci Rep 11:5618
Bryaskova R, Pencheva D, Nikolov S, Kantardjiev T (2011) Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP). J Chem Biol 4:185–191. https://doi.org/10.1007/s12154-011-0063-9
Fanta GF, Kenar JA, Felker FC, Byars JA (2013) Preparation of starch-stabilized silver nanoparticles from amylose-sodium palmitate inclusion complexes. Carbohydr Polym 92:260–268. https://doi.org/10.1016/j.carbpol.2012.09.016
Yang Z, Peng H, Wang W, Liu T (2010) Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J Appl Polym Sci 116:2658–2667. https://doi.org/10.1002/app
Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, Thangamani S (2012) Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed 2:574–580. https://doi.org/10.1016/S2221-1691(12)60100-2
Mohamed F, Enaiet Allah A, Abu Al-Ola KA, Shaban M (2021) Design and characterization of a novel ZnO–Ag/polypyrrole core–shell nanocomposite for water bioremediation. Nanomaterials 11:1688
Rajarajan K, Anandhi A, Reka KRD, Madhurambal G (2013) Synthesis and characterization studies of diphenylamine picrate cystal–a non-linear optical material. Interactions 9:10
Shaban M, Mohamed F, Abdallah S (2018) Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst. Sci Rep 8:1–15. https://doi.org/10.1038/s41598-018-22324-7
Mohamed F, Abukhadra MR, Shaban M (2018) Removal of safranin dye from water using polypyrrole nanofiber/Zn-Fe layered double hydroxide nanocomposite (Ppy NF/Zn-Fe LDH) of enhanced adsorption and photocatalytic properties. Sci Total Environ 640–641:352–363. https://doi.org/10.1016/j.scitotenv.2018.05.316
Kalaivani R, Maruthupandy M, Muneeswaran T, Hameedha Beevi A, Anand M, Ramakritinan CM et al (2018) Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front Lab Med 2:30–35. https://doi.org/10.1016/j.flm.2018.04.002
Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M (2004) Biological synthesis of triangular gold nanoprisms. Nat Mater 3:482–488. https://doi.org/10.1038/nmat1152
Kurowiak J, Kaczmarek-Pawelska A, Mackiewicz A, Baldy-Chudzik K, Mazurek-Popczyk J, Zaręba Ł, Klekiel T, Będziński R (2021) Synthesis, characterization and antimicrobial activity applications of grafted copolymer alginateg-poly(N-vinyl imidazole). RSC Adv 11:11541
Ehsan NK, Wenwen G, Xin W, Weiyi X, Lei S, Yuan H (2020) Effect of metal-based nanoparticles decorated graphene hybrids on flammability of epoxy nanocomposites. Compos Part A: Appl Sci Manuf 129:105694
Gokhan A, Cagatay A, Hulya OL, Mehmet AT (2018) Antibacterial film from chlorinated polypropylene via CuAAC click chemistry. Progr Organ Coat 125:73–78
Cagatay A, Gokhan A, Ozgun D, Hakan D, Ilknur T, Esra A (2022) A facile approach for the fabrication of antibacterial nanocomposites: a case study for AgNWs/poly(1,4-cyclohexanedimethylene acetylene dicarboxylate) composite networks by aza-Michael addition. Eur Polym J 169(15):111130
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, F., Ahmed, A. & Abdel-Gawad, O.F. Preparation and evaluation of the antimicrobial activity of sodium alginate-grafted diphenylamine embedded with silver nanoparticles. Polym. Bull. 80, 9797–9810 (2023). https://doi.org/10.1007/s00289-022-04505-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04505-7