Abstract
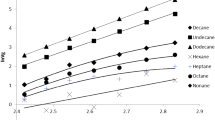
In this work, polyvinyl butyral (PVB) with number-average molecular weight of 35,700 g mol−1 and butyraldehyde content of 78.66% was synthesized, and was characterized by Fourier transform infrared spectroscopy (FTIR), gel permeation chromatography (GPC), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), derivative thermogravimetric analysis (DTG) and pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS). The results showed that the prepared PVB has high degree of acetalization and high thermal stability. Hansen solubility parameters (HSPs) of PVB were determined by HSPiP software, turbidimetric titration, intrinsic viscosity method and group contribution method. The Flory–Huggins interaction parameters (χ and χHSP) between PVB and solvents were calculated, and the results showed that the intrinsic viscosity [η] increased linearly with the decrease of χ and χHSP values. Both the graph of [η] versus χ and the graph of [η] versus χHSP can be divided into dissolving area and nondissolving area by χ = 0.5 and χHSP = 0.5, which fully proved that the HSPs obtained in this paper were convincing. It is expected that these parameters are available for predicting the solubility of PVB in various solvents and the compatibility with additives.













Similar content being viewed by others
Data availability
All data and materials support published claims and comply with field standards.
References
Lin XY, Wang K, Zhang JS, Luo GS (2015) Process intensification of the synthesis of poly(vinyl butyral) using a microstructured chemical system. Ind Eng Chem Res 54:3582–3588. https://doi.org/10.1021/acs.iecr.5b00911
Lian F, Wen Y, Ren Y, Guan H (2014) A novel PVB based polymer membrane and its application in gel polymer electrolytes for lithium-ion batteries. J Membr Sci 456:42–48. https://doi.org/10.1016/j.memsci.2014.01.010
Zhang P, Wang Y, Xu Z, Yang H (2011) Preparation of poly (vinyl butyral) hollow fiber ultrafiltration membrane via wet-spinning method using PVP as additive. Desalination 278:186–193. https://doi.org/10.1016/j.desal.2011.05.026
Ma X, Sun Q, Su Y, Wang Y, Jiang Z (2007) Antifouling property improvement of poly(vinyl butyral) ultrafiltration membranes through acid treatment. Sep Purif Technol 54:220–226. https://doi.org/10.1016/j.seppur.2006.09.006
Ruediger T, Berg A, Guellmar A, Rode C, Schnabelrauch M, Urbanek A, Wagner K, Wyrwa R, Kinne RW, Sigusch BW (2012) Cytocompatibility of polymer-based periodontal bone substitutes in gingival fibroblast and MC3T3 osteoblast cell cultures. Dent Mater 28:239–249. https://doi.org/10.1016/j.dental.2012.05.008
Posavec D, Dorsch A, Bogner U, Bernhardt G, Nagl S (2011) Polyvinyl butyral nanobeads: preparation, characterization, biocompatibility and cancer cell uptake. Microchim Acta 173:391–399. https://doi.org/10.1007/s00604-011-0573-8
Kraft A, Rottmann M (2009) Properties, performance and current status of the laminated electrochromic glass of Gesimat. Sol Energy Mater Sol Cells 93:2088–2092. https://doi.org/10.1016/j.solmat.2009.05.010
Liu H, Feng Y, Wang Z, Wang K, Xie J (2008) A PVB-based rheological phase approach to nano-LiFePO4/C composite cathodes. Powder Technol 184:313–317. https://doi.org/10.1016/j.powtec.2007.09.002
Zhou BY, Lin XY, Wang K, Luo GS (2017) Technology for an energy-saving and fast synthesis of polyvinyl butyral in a microreactor system. Ind Eng Chem Res 56:14463–14470. https://doi.org/10.1021/acs.iecr.7b03906
Zhou X, Sun K, Gao J, Le S, Zhang N, Wang P (2009) Microstructure and electrochemical characterization of solid oxide fuel cells fabricated by co-tape casting. J Power Sources 191:528–533. https://doi.org/10.1016/j.jpowsour.2009.02.008
Thejo Kalyani N, Dhoble SJ (2012) Organic light emitting diodes: Energy saving lighting technology–a review. Renew Sustain Energy Rev 16:2696–2723. https://doi.org/10.1016/j.rser.2012.02.021
Dhaliwal AK, Hay JN (2002) The characterization of polyvinyl butyral by thermal analysis. Thermochim Acta 391:245–255
Zhang XH, Yang GL, Hu JQ, Zhang CY (2013) Preparation of alcohol-soluble polyvinyl butyral. Adv Mater Res 750–752:1831–1835. https://doi.org/10.4028/www.scientific.net/AMR.750-752.1831
Hildebrand JH (1949) A critique of the theory of solubility of non-electrolytes. Chem Rev 44:37–45
Hildebrand JH (1965) Order from chaos. Science 150:441–450
Hansen CM (2007) Solubility parameters–an introduction. Hansen solubility parameters: a user’s handbook. CRC Press, Boca Raton, pp 4–9
Lin X, Yan S, Zhou B, Wang K, Zhang J, Luo G (2017) Highly efficient synthesis of polyvinyl butyral (PVB) using a membrane dispersion microreactor system and recycling reaction technology. Green Chem 19:2155–2163. https://doi.org/10.1039/c7gc00670e
Yang B, Liu R, Huang J, Sun H (2013) Reverse dissolution as a route in the synthesis of poly(vinyl butyral) with high butyral contents. Ind Eng Chem Res 52:7425–7431. https://doi.org/10.1021/ie400559s
Fernández MD, Fernández MJ, Hoces P (2006) Synthesis of poly(vinyl butyral)s in homogeneous phase and their thermal properties. J Appl Polym Sci 102:5007–5017. https://doi.org/10.1002/app.25004
Fernández MD, Fernández MJ, Hoces P (2008) Poly(vinyl acetal)s containing electron-donor groups: Synthesis in homogeneous phase and their thermal properties. React Funct Polym 68:39–56. https://doi.org/10.1016/j.reactfunctpolym.2007.10.012
Hansen CM (2007) Methods of characterization-polymers. Hansen solubility parameters: a user’s handbook. CRC Press, Boca Raton, pp 99–106
Hansen CM (2007) Appendix A. Hansen solubility parameters: a user’s handbook. CRC Press, Boca Raton, pp 345–483
Suh KW, Clarke DH (1967) Cohesive energy densities of polymers from turbidimetric titrations. J Polym Sci, Part A-1 Polym Chem 5:1671–1681
Suh KW, Corbet JM (1968) Solubility parameters of polymers from turbidimetric titrations. J Polym Sci, Part A-1: Polym Chem 12:2359–2370
Boucher DS (2020) Solubility parameters and solvent affinities for polycaprolactone: a comparison of methods. J Appl Polym Sci 137:48908–48920. https://doi.org/10.1002/app.48908
Segarceanu O, Leca M (1997) Improved method to calculate Hansen solubility parameters of a polymer. Prog Org Coat 31:307–310
Van Krevelen DW, Te Nijenhuis K (2009) Cohesive properties and solubility. Properties of polymers. Elsevier, Amsterdam, pp 213–220
Stefanis E, Panayiotou C (2008) Prediction of hansen solubility parameters with a new group-contribution method. Int J Thermophys 29:568–585. https://doi.org/10.1007/s10765-008-0415-z
Easley AD, Vukin LM, Flouda P, Howard DL, Pena JL, Lutkenhaus JL (2020) Nitroxide radical polymer-solvent interactions and solubility parameter determination. Macromolecules 53:7997–8008. https://doi.org/10.1021/acs.macromol.0c01739
Schenderlein S, Luck M, Muller BW (2004) Partial solubility parameters of poly(D, L-lactide-co-glycolide). Int J Pharm 286:19–26. https://doi.org/10.1016/j.ijpharm.2004.07.034
Qin X-X, Cheng Z-L (2016) Application of ionic liquids as a catalyst in the synthesis of polyvinyl butyral (PVB) polymer. Chin Chem Lett 27:145–148. https://doi.org/10.1016/j.cclet.2015.07.012
Bai YK, Chen Y, Wang QH, Wang TM (2014) Poly(vinyl butyral) based polymer networks with dual-responsive shape memory and self-healing properties. J Mater Chem A 2:9169–9177. https://doi.org/10.1039/c4ta00856a
Hoepfner JC, Loos MR, Pezzin SH (2019) Role of the degree of acetalization on dynamic mechanical properties of polyvinyl butyral/carbon nanotube composites. J Appl Polym Sci. https://doi.org/10.1002/app.48146
Liau LCK, Yang TCK, Viswanath DS (1996) Reaction pathways and kinetic analysis of PVB thermal degradation using TG/FT-IR. Appl Spectrosc 50:1058–1065
Kingston GC, Yuen HK (1987) Application of evolved gas analysis to the study of poly(vinyl butyral) thermal stability. Thermochim Acta 116:317–327. https://doi.org/10.1016/0040-6031(87)88192-3
Bordes C, Freville V, Ruffin E, Marote P, Gauvrit JY, Briancon S, Lanteri P (2010) Determination of poly(epsilon-caprolactone) solubility parameters: application to solvent substitution in a microencapsulation process. Int J Pharm 383:236–243. https://doi.org/10.1016/j.ijpharm.2009.09.023
Hansen CM (2007) Theory-the prigogine corresponding states theory, χ12 interaction parameter, and hansen solubility parameters. Hansen solubility parameters: a user’s handbook. CRC Press, Boca Raton, pp 27–33
Wang YZ, Bi LY, Zhang HJ, Zhu XT, Liu GY, Qiu GX, Liu SS (2019) Predictive power in oil resistance of fluororubber and fluorosilicone rubbers based on three-dimensional solubility parameter theory. Polym Test 75:380–386. https://doi.org/10.1016/j.polymertesting.2019.03.004
Liu SS, Li XP, Qi PJ, Song ZJ, Zhang Z, Wang K, Qiu GX, Liu GY (2020) Determination of three-dimensional solubility parameters of styrene butadiene rubber and the potential application in tire tread formula design. Polym Test 81:106170–106174. https://doi.org/10.1016/j.polymertesting.2019.106170
Lindvig T, Michelsen ML, Kontogeorgis GM (2002) A Flory-Huggins model based on the Hansen solubility parameters. Fluid Phase Equilib 203:247–260
Flory PJ (1942) Thermodynamics of high polymer solutions. J Chem Phys 10:51–61. https://doi.org/10.1063/1.1723621
Flory PJ, Rehner J (1943) Statistical mechanics of cross-linked polymer networks II. Swelling J Chem Phys 11:521–526. https://doi.org/10.1063/1.1723792
Acknowledgements
Financial support under the research grant 420094 from Sinopec Shanghai Research Institute of Petrochemical Technology is acknowledged.
Funding
This work was supported by Sinopec Shanghai Research Institute of Petrochemical Technology under Grant 420094.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunyu Wang and Wenwen Luan contributed equally to this work and should be considered co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, C., Luan, W., Zeng, Z. et al. Synthesis, solvent interactions and Hansen solubility parameters of polyvinyl butyral. Polym. Bull. 80, 6363–6383 (2023). https://doi.org/10.1007/s00289-022-04366-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04366-0