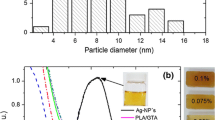
Abstract
Blends of poly(L-lactic acid)/nanoparticle silver and GTA (plasticizer) are present, and the crystallization effect was studied. The effect of the crystallization was investigated by oxygen permeability, isothermal crystallization, and mechanical behavior. Observed properties varied according to the concentration of Ag-NP (0.025–0.1%) dispersed in the continuous phase PLLA. Overall isothermal crystallization rates of the PLLA nanocomposites were higher than those of neat PLLA due to the nucleating effect of the Ag-NP and the enhanced chain mobility caused by the plasticizer (GTA). Ternary blends exhibited an improvement in toughness compared to PLLA alone. Also, the effect is due to the α and αʹ crystals growing up, as observed by XRD. The films exhibited a high barrier of oxygen permeability from 2118 to 18 cc·mm/m2·d, 99% lower than that of PLLA (or 117 times lower), Ag-NP concentration (0.1%) dispersed in the polymer with GTA as a plasticizer.










Similar content being viewed by others
References
Rasal R, Janorkar A, Hirt D (2010) Poly(lactic acid) modifications. Prog Polym Sci 35:338–356. https://doi.org/10.1016/j.progpolymsci.2009.12.003
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromol Biosci 4:835–864. https://doi.org/10.1002/mabi.200400043
De Santis F, Pantani R, Titomanlio G (2011) Nucleation and crystallization kinetics of poly(lactic acid). Thermochim Acta 522:128–134. https://doi.org/10.1016/j.tca.2011.05.034
Kalb B, Pennings A (1980) General crystallization behaviour of poly(l-lactic acid). Polymer 21:607–612. https://doi.org/10.1016/0032-3861(80)90315-8
Cocca M, Di Lorenzo M, Malincolico M, Frezza V (2011) Influence of crystal polymorphism on mechanical and barrier properties of poly(L-lactic acid. Eur Polym J 47:1073–1080. https://doi.org/10.1016/j.eurpolymj.2011.02.009
Saiedlou S, Huneault M, Li H, Park C (2012) Poly(lactic acid) crystallization. Prog Polym Sci 37:1657–1677. https://doi.org/10.1016/j.progpolymsci.2012.07.005
Zhang J, Tashiro K, Tsuji H, Domb A (2008) Disorder-to-order phase transition and multiple melting behavior of Poly(lactic acid) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 41:1352–1357. https://doi.org/10.1021/ma0706071
Righetti M, Gazzano M, Di Lorenzo M, Androsch R (2015) Enthalpy of melting of α′-and α-crystals of poly (l-lactic acid). Eur Polym J 70:215–220. https://doi.org/10.1016/j.eurpolymj.2015.07.024
Tang Z, Zhang C, Liu X, Zhu J (2012) The crystallization behavior and mechanical properties of polylactic acid in the presence of a crystal nucleating agent. J Appl Polym Sci 125:1108–1115. https://doi.org/10.1002/app.34799
Courgneau C, Ducruet V, Averous L, Grenet J, Domenek S (2013) Nonoisothermal crystallization kinects of poly(lactide)-Effect of plasticizers and nucleating agent. Polym Eng Sci 53:1085–1098. https://doi.org/10.1002/pen.23357
Shi X, Zhang G, Vu Phuong T, Lazzeri A (2015) Synergistic effects of nucleating agents and plasticizers on the crystallization behavior of poly(lactic acid). Molecules 20:1579–1593. https://doi.org/10.3390/molecules20011579
Li M, Hu D, Wang Y, Shen C (2010) Nonisothermal crystallization kinetics of poly(lactic acid) formulations comprising talc with poly(ethylene glycol). Polym Eng Sci 50:2298–2305. https://doi.org/10.1002/pen.21755
Karami S, Lafleur P (2015) Role of chain dynamics and topological confinements in cold crystallization of PLA-clay nanocomposites. Polym Eng Sci 55:1310–1320. https://doi.org/10.1002/pen.24070
Piorkowska E, Kulinski Z, Galeski A, Masirek R (2006) Plasticization of semicrystalline poly(L-lactide) with poly(propylene glycol). Polymer 47:7178–7188. https://doi.org/10.1016/j.polymer.2006.03.115
Salas-Papayanopolos H, Morales-Cepeda A, Sanchez S, Lafleur P, Gomez I (2017) Synergistic effect of silver nanoparticle content on the optical and thermo-mechanical properties of poly(L-lactic acid)/glycerol triacetate blends. Polym Bull 74:4799–4814. https://doi.org/10.1007/s00289-017-1992-4
Herrera N, Mathew A, Oksman K (2015) Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: mechanical, thermal and optical properties. Compos Sci Technol 106:149–155. https://doi.org/10.1016/j.compositesa.2015.05.024
Oskman K, Skrifvars M, Selin J (2003) Natural fibres as reinforcement in polylactic acid (PLA) composites. Comp Sci and Tech 63:1317–1324. https://doi.org/10.1016/S0266-3538(03)00103-9
An J, Tang B, Ning X, Zhou J, Xu S, Zhao B, Xu W, Corredor C, Lombardi J (2007) Photoinduced shape evolution: from triangular to hexagonal silver nanoplates. J Phys Chem C 111:18055–18059. https://doi.org/10.1021/jp0745081
Kelly K, Coronado E, Zhao L, Schatz G (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668–677. https://doi.org/10.1021/jp026731y
Van Dong P, Ha C, Binh L (2012) Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. J Int Nano Lett 2:9. https://doi.org/10.1186/2228-5326-2-9
Shameli K, Bin Ahmad M, Wan Yunus W, Ibrahim N, Rahman R, Jokar M, Darroudi M (2010) Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int J Nanomed 5:573–579. https://doi.org/10.2147/IJN.S13227
Gorrasi G, Sorrentino A, Pantani R (2015) Modulation of biodegradation rate of poly(lactic acid) by silver nanoparticles. J Polym Environ 23:316–320. https://doi.org/10.1007/s10924-015-0720-0
Anakabe J, Zaldua Huici A, Eceiza A, Arbelaiz A, Avérous L (2017) Combined effect of nucleating agent and plasticizer on the crystallization behaviour of polylactide. Polym Bull 1:1–30. https://doi.org/10.1007/s00289-017-1989-z
Mark H (2004) Encyclopedia of polymer science and technology. Willey, USA
Sperling L (2006) Introduction to physical polymer science. Willey, USA
Lim LT, Auras R, Rubino M (2008) Processing technologies for poly(lactic acid). Prog Polym Sci 33:820–852. https://doi.org/10.1016/j.progpolymsci.2008.05.004
Vodnik V, Bozanic DK, Dzunuzovic J, Vukoje I, Nedeljkovic J (2012) Silver/polystyrene nanocomposites: optical and thermal properties. Polym Compos 33:782–788. https://doi.org/10.1002/pc.22207
Tjong SC, Bao S (2007) Structure and mechanical behavior of isotactic polypropylene composites filled with silver nanoparticles. E-Polymers 139:139–155. https://doi.org/10.1515/epoly.2007.7.1.1618
Shieh Y, Liu G (2007) Temperature-modulated differential scanning calorimetry studies on the origin of double melting peaks in isothermally melt-crystallized poly(L-lactic acid). J Polym Sci B Poly Phy 45:466–474. https://doi.org/10.1002/polb.21056
Turner J, Riga A, O’Connor A, Zhang J, Collis J (2004) Characterization of drawn and undrawn poly- L -lactide films by differential scanning calorimetry. J Therm Anal Calorim 75:257–268. https://doi.org/10.1023/B:JTAN.0000017347.08469.b1
Huang S, Li H, Jiang S, Chen X, An L (2011) Crystal structure and morphology influenced by shear effect of poly(l-lactide) and its melting behavior revealed by WAXD, DSC and in-situ POM. Polymer 52:3478–3487. https://doi.org/10.1016/j.polymer.2011.05.044
Androsch R, Schick C, Di Lorenzo M (2014) Melting of conformationally disordered crystals (α′-Phase) of poly(l-lactic acid). Macromol Chem Physic 215:1134–1139. https://doi.org/10.1002/macp.201400126
Avrami M (1940) Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J Chem Phys 8:212–224. https://doi.org/10.1063/1.1750631
Avrami M (1941) Granulation, phase change, and microstructure kinetics of phase change. III J Chem Phys 9:177–184. https://doi.org/10.1063/1.1750872
Lorenzo A, Arnal M, Albuerne J, Müller A (2007) DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym Test 26:222–231. https://doi.org/10.1016/j.polymertesting.2006.10.005
Xiao H, Ren X, Jiang T, Yeh J (2010) Isothermal crystallization kinetics and crystal structure of poly(lactic acid): Effect of triphenyl phosphate and talc. J Appl Polym Sci 118:3558–3569. https://doi.org/10.1002/app.32728
Hermans PH, Weidinger A (1961) On the determination of the crystalline fraction of polyethylenes from X-ray diffraction. Die Makromol Cheme 44:24–36. https://doi.org/10.1002/macp.1961.020440103
Chen X, Han L, Zhang T, Zhan J (2014) Influence of crystal polymorphism on crystallinity calculation of poly(L-lactic acid) by infrared spectroscopy. Vib Spectrosc 70:1–5. https://doi.org/10.1016/j.vibspec.2013.10.003
Acknowledgements
Authors are grateful to Kees Joziasse who provides the PLLA from Corbion Purac. This work was supported by the Consejo Nacional de Ciencia y Tecnología [CONACyT, grant number 220989] and Tecnológico Nacional de México [TNM]. Hernán Peraza-Vazquez would like to express his gratitude to Instituto Politécnico Nacional.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salas-Papayanopolos, H., Morales-Cepeda, A.B., Wood-Adams, P. et al. Crystallization effect of poly(L-lactic acid)/silver nanocomposites blends, on barrier and mechanical properties using glyceryl triacetate as plasticizer. Polym. Bull. 80, 5273–5290 (2023). https://doi.org/10.1007/s00289-022-04309-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04309-9