Abstract
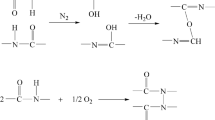
In this paper, the polycondensation kinetics of bio-based semi-aromatic high-temperature polyamide PA5T/56 was studied in a micro-high-temperature and high-pressure reactor. The polycondensation experiment of PA5T/56 salt solution with temperature of 240–300 ℃ and initial water content of 5–30wt% was carried out. The change of end group content in the reaction process was investigated, and the relationship between polymer equilibrium molecular weight and temperature and water content was explored. The results showed that the end group concentration decreased rapidly at the beginning of the reaction and approached equilibrium after a period of time, and the higher the temperature, the higher the initial water content and the lower the equilibrium molecular weight, indicating that the polycondensation of PA5T/56 was an exothermic reaction; on this basis, a kinetics model of polycondensation reaction was established based on the literature model, it was considered that the polycondensation reaction is an acid-catalyzed third-order reaction, and the effects of temperature and water content on the equilibrium and rate of polycondensation reaction can be investigated. The kinetics model of polycondensation reaction was obtained, and the calculated results of the model were basically consistent with the experimental values.




Similar content being viewed by others
References
Chuanhui Z, Kancheng M, Min C (2012) Research progress in heat-resistant nylon. Eng Plast Appl 11(2):33–35
Blondel P, Briffaud T, Werth MRG (1997) Kinetics of polycondensation in an industrial environment. Macromol Symp 122(1):243–248
Mallon FK, RAY WH (2015) A comprehensive model for nylon melt equilibria and kinetics. J Appl Polym Sci 69(6):1213–1231
Ghasem N, Ghasem N (2014) Polymerization of hot melt adhesives from dicarboxylic fatty acid for introductory organic chemistry laboratories. J Lab Chem Educ 2(5):85–89
Kale V, Vijayalakshmi P, Rao TC (1989) Kinetics of reaction of C21 cycloaliphatic dicarboxylic acid and ethylenediamine. J Appl Polym Sci 38(7):1287–1293
Heidarian J, Ghasem N, Daud WAW (2005) Kinetics of polymerization of dimer fatty acids with ethylenediamine after 90% conversion. Macromol Chem Phys 206(6):658–663
Schaffer MA, Mcauley KB, Cunningham MF, Marchildon EK (2003) Experimental study and modeling of nylon polycondensation in the melt phase. Ind Eng Chem Res 42(13):2946–2959
Sánchez-Jiménez PE , Perejón A, Arcenegui-Troya J, Pérez-Maqueda LA (2021) Predictions of polymer thermal degradation: relevance of selecting the proper kinetic model. J Therm Anal Calorim 147:2335–2341
Hu Z, WyrzykowskiLura MP (2020) Estimation of reaction kinetics of geopolymers at early ages. Cem Concr Res 129:105971–105973
Jin C, Suehiro T, Kodama A, Goto M, Hirose T (2001) Equilibrium and reaction rate of reversible BaO–BaO2 reaction for oxygen production. J Chem Eng Jpn 34(2):279–282
Gregory SA, Li Y, Monroe TD, Li J, Losego MD (2021) Vapor phase infiltration doping of the semiconducting polymer poly(aniline) with TiCl4+H2O: mechanisms, reaction kinetics, and electrical and optical properties. ACS Appl Polym Mater 3(2):720–729
Rl A, Lh A, Jb A, Vf B (2020) A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol 153:303–310
So HL, Chu W, Wang YH (2019) Naphthalene degradation by Fe2+/Oxone/UV: applying an unconventional kinetics model and studying the reaction mechanism. Chemosphere 218:110–118
Hosseini S, Brake NA, Nikookar M, Günaydn-En Z, Snyder HA (2021) Mechanochemically activated bottom ash-fly ash geopolymer. Cem Concr Compos 6:103976
Mallon FK, Ray WH (1998) A comprehensive model for nylon melt equilibria and kinetics. J Appl Polym Sci 69(6):1213–1231
Fukumoto O (1961) Equilibria between polycapramide and water. J Polym Sci 22(101):263–270
Ogata N (1961) Studies on polycondensation reactions of nylon salt II. The rate of polycondensation reaction of nylon 66 salt in the presence of water. Die Makromolekulare Chemie Macromol Chem Phys 43(1):117–131
Acknowledgements
We thank all those who participated in the study. This research was supported by Shanghai Cathay Biotechnology Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, C., Liu, X. Study on reaction kinetics of bio-based semi-aromatic high-temperature polyamide PA5T/56. Polym. Bull. 80, 2503–2514 (2023). https://doi.org/10.1007/s00289-022-04176-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04176-4