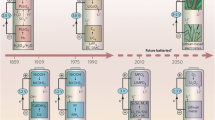
Abstract
Energy is an essential factor in our day-to-day life. The major demand for energy in modern society has been increasing rapidly. Among all energy storage systems, batteries are one of the most efficient devices. Li-ion batteries have received huge attention due to their unique characteristics like high energy density, flexibility, lightweight, and a longer lifespan than comparable battery technologies. Solid polymer electrolytes have gained huge attention in high-performance LIB due to their high safety, no leakage, wide electrochemical stability window, mechanical flexibility, and thermal stability. In this review, a series of polymers such as PMMA, PEO, PAN, PVDF, PVDF-HFP, PVC, PVA, PS, and PC are discussed. Some of the recent publications of polymer electrolyte with different combinations of lithium salt are highlighted. The characteristics and drawbacks of commonly used lithium salt are listed. In order to improve the ionic conductivity, various salt, solvents, and inorganic filler/clay are used. This review opens doors for novel approaches and also provides the progress of new solid polymer electrolytes.
Graphical abstract










Similar content being viewed by others
References
Michel A (1990) Polymers with ionic conductivity. Adv Mater 2:278–286
Michel Armand B (1986) Polymer electrolytes. Annu Rev Mater Sci 16:245–261
Sequeira CAC, Santos DMF (2010) Introduction to polymer electrolyte materials. Polym Electrolytes Fundam Appl. https://doi.org/10.1533/9781845699772.1.3
Di Noto V, Lavina S, Giffin GA et al (2011) Polymer electrolytes: present, past and future. Electrochim Acta 57:4–13. https://doi.org/10.1016/j.electacta.2011.08.048
Blumberg AA, Pollack SS, Hoeve CAJ (1964) A poly(ethylene oxide)–mercuric chloride complex. J Polym Sci Part A Gen Pap 2:2499–2502. https://doi.org/10.1002/pol.1964.100020601
Moacanin J, Cuddihy EF (2007) Effect of polar forces on the viscoelastic properties of poly(propylene oxide). J Polym Sci Part C Polym Symp 14:313–322. https://doi.org/10.1002/polc.5070140124
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science (80- ) 334:928–935. https://doi.org/10.1126/science.1212741
Sequeira CAC, Santos DMF (2010) Polymer electrolytes: fundamentals and applications. Polym Electrolytes Fundam Appl. https://doi.org/10.1533/9781845699772
Armand M (1983) Polymer solid electrolytes–an overview. Solid State Ionics 9–10:745–754. https://doi.org/10.1016/0167-2738(83)90083-8
Wright PV (1989) Recent trends in polymer electrolytes based on polyethylene oxide). J Macromol Sci Part A–Chem 26:519–550. https://doi.org/10.1080/00222338908051991
Armand M (1994) The history of polymer electrolytes. Solid State Ionics 69:309–319. https://doi.org/10.1016/0167-2738(94)90419-7
Fenton DE, Parker JM, Wright PV (1973) Complexes of alkali metal ions with poly(ethylene oxide). Polymer (Guildf) 14:589. https://doi.org/10.1016/0032-3861(73)90146-8
Wright PV (1975) Electrical conductivity in ionic complexes of poly(ethylene oxide). Br Polym J 7:319–327. https://doi.org/10.1002/pi.4980070505
Gray F, Armand M (2011) Polymer Electrolytes. Handb Batter Mater Second Ed. https://doi.org/10.1002/9783527637188.ch18
Venkatasetty H V. (2000) Lithium-polymer electrolyte rechargeable batteries. Proc Annu Batter Conf Appl Adv 2000-Janua:109–114. https://doi.org/10.1109/BCAA.2000.838389
David Linden TB (2002) Handbook of Batteries, 3rd edn. McGraw-Hill
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367. https://doi.org/10.1038/35104644
O’Heir J (2017) Building better batteries. Mech Eng 139:10–11
Long L, Wang S, Xiao M, Meng Y (2016) Polymer electrolytes for lithium polymer batteries. J Mater Chem A 4:10038–10039. https://doi.org/10.1039/c6ta02621d
Wright PV (1998) Polymer electrolytes–the early days. Electrochim Acta 43:1137–1143. https://doi.org/10.1016/S0013-4686(97)10011-1
Meyer WH (1998) Polymer electrolytes for Lithium ion batteries. Adv Mater 10:439–448
Warner JT (2019) Lithium-Ion Battery Chemistries A Primer. Elsevier
H T (2000) portable Li-ion worldwide. Conf Proc power
Armand M, Tarascon JM (2008) Building better batteries. Nature 451(7179):652–657
R.K A r. and G, 1999 Superionic solid composite electrolyte phase - An overview J Mater Sci 34:1131–1162
Appetecchi GB, Scrosati B (1998) Lithium ion polymer battery. Electrochim Acta 43:1105–1107. https://doi.org/10.1016/S0013-4686(97)10117-7
Kim JY, Kim SH (1999) Ionic conduction behavior of network polymer electrolytes based on phosphate and polyether copolymers. Solid State Ionics 124:91–99. https://doi.org/10.1016/S0167-2738(99)00104-6
Kang Xu (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteriesno title. Chem Rev 104:4303–4417
Winter M, Besenhard JO, SpahrA ME, Novak P (1998) Insertion electrode materials for rechargeable lithium batteries. Adv Mater 10:725–763
Kasnatscheew J, Wagner R, Winter M, Cekic-Laskovic I (2018) Interfaces and materials in lithium ion batteries: challenges for theoretical electrochemistry. Top Curr Chem 376:1–29. https://doi.org/10.1007/s41061-018-0196-1
Fong R, von Sacken U, Dahn JR (1990) Studies of lithium intercalation into carbons using nonaqueous electrochemical cells. J Electrochem Soc 137:2009–2013. https://doi.org/10.1149/1.2086855
Kasnatscheew J, Placke T, Streipert B et al (2017) A tutorial into practical capacity and mass balancing of lithium ion batteries. J Electrochem Soc 164:A2479–A2486. https://doi.org/10.1149/2.0961712jes
Chawla N, Bharti N, Singh S (2019) Recent advances in non-flammable electrolytes for safer lithium-ion batteries. Batteries 5(1):19. https://doi.org/10.3390/batteries501001
Wang WM (2012) Study on all solid-state composite polymer electrolyte. Adv Mater Res 571:13–16. https://doi.org/10.4028/www.scientific.net/AMR.571.13
Vincent CA (1994) Applications of electroactive polymers. Edited by B. Scrosati. Chapman and Hall, London, 1993. pp. 354 price £40.00. ISBN 0–412–41430–9. Polym Int 33:343–343. https://doi.org/10.1002/pi.1994.210330323
Scrosati B, Hassoun J, Sun YK (2011) Lithium-ion batteries. A look into the future. Energy Environ Sci 4:3287–3295. https://doi.org/10.1039/c1ee01388b
Janek J, Zeier WG (2016) A solid future for battery development. Nat Energy 1:1–4. https://doi.org/10.1038/nenergy.2016.141
Mark A. Ratner, Patrik Johansson and, Duward F. Shriver (2000) Polymer Electrolytes: Ionic Transport Mechanisms and Relaxation Coupling. Mrs Bull 31–37
Ye L, Feng Z (2010) Polymer electrolytes as solid solvents and their applications. Polym Electrolytes Fundam Appl. https://doi.org/10.1533/9781845699772.2.550
Randau S, Weber DA, Kötz O et al (2020) Benchmarking the performance of all-solid-state lithium batteries. Nat Energy 5:259–270. https://doi.org/10.1038/s41560-020-0565-1
Homann G, Stolz L, Neuhaus K et al (2020) Effective optimization of high voltage solid-state lithium batteries by using poly(ethylene oxide)-based polymer electrolyte with semi-interpenetrating network. Adv Funct Mater 30:1–8. https://doi.org/10.1002/adfm.202006289
Mauger A, Julien CM, Paolella A et al (2019) Building better batteries in the solid state: a review. Materials (Basel) 12:1–86. https://doi.org/10.3390/ma122333892
Lr G (1993) Appliction of electroactive polymers. First. Chapman & Hall, London
Quartarone E, Mustarelli P (2011) Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem Soc Rev 40:2525–2540. https://doi.org/10.1039/c0cs00081g
Farrington GC, Briant JL (1979) Fast Ionic Transport in Solids Authors ( s ): Gregory C . Farrington and Jacqueline L . Briant Published by : American Association for the Advancement of Science Stable URL : https://www.jstor.org/stable/1748409 Accessed : 27–03–2016 08 : 22 UTC. Science (80- ) 204:1371–1379
Pandey GP, Hashmi SA, Agrawal RC (2008) Experimental investigations on a proton conducting nanocomposite polymer electrolyte. J Phys D Appl Phys. https://doi.org/10.1088/0022-3727/41/5/055409
Zhang Q, Liu K, Ding F, Liu X (2017) Recent advances in solid polymer electrolytes for lithium batteries. Nano Res 10:4139–4174. https://doi.org/10.1007/s12274-017-1763-4
Killis A, LeNest J-F, Cheradame H, Gandini A (1982) Ionic conductivity of polyether-polyurethane networks containing NaBPh4: a free volume analysis. Die Makromol Chemie 183:2835–2845. https://doi.org/10.1002/macp.1982.021831118
Weston JE, Steele BCH (1982) Effects of preparation method on properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ionics 7:81–88. https://doi.org/10.1016/0167-2738(82)90073-X
Leveque M, Le Nest JF, Gandini A, Cheradame H (1985) Cationic transport numbers in polyether-based networks containing lithium salts. J Power Sources 14:27–30. https://doi.org/10.1016/0378-7753(85)88006-X
Bouridah A, Dalard F, Deroo D, Armand MB (1986) Potentiometric measurements of ionic mobilities in poly(ethyleneoxide) electrolytes. Solid State Ionics 18–19:287–290. https://doi.org/10.1016/0167-2738(86)90128-1
W GoreckiR AndreaniC. BerthierM. ArmandM. MaliJ. RoosD. Brinkmann 1986 NMR, DSC and conductivity study of poly(ethylene oxide) complex electrolyte: PEO (LiClO4)X Solid State Ionics 18 295 299
Zugmann S, Fleischmann M, Amereller M et al (2011) Measurement of transference numbers for lithium ion electrolytes via four different methods, a comparative study. Electrochim Acta 56:3926–3933. https://doi.org/10.1016/j.electacta.2011.02.025
Fauteux D, Boisvert Y, Robitaille CD, Lupien MD (1985) Phase Diagram, Conductivity and Transference Number of Poly(Ethylene Oxide) Base Electrolytes. Electrochem Soc Ext Abstr 85–2:189
Zhou X, Li X, Li Z et al (2021) Hybrid electrolytes with an ultrahigh Li-ion transference number for lithium-metal batteries with fast and stable charge/discharge capability. J Mater Chem A 9:18239–18246. https://doi.org/10.1039/d1ta04631d
Devaux D, Leduc H, Dumaz P et al (2020) Effect of electrode and electrolyte thicknesses on all-solid-state battery performance analyzed with the sand equation. Front Energy Res. https://doi.org/10.3389/fenrg.2019.00168
Ehrl A, Landesfeind J, Wall WA, Gasteiger HA (2017) Determination of transport parameters in liquid binary electrolytes: part II. Transf Number J Electrochem Soc 164:A2716–A2731. https://doi.org/10.1149/2.1681712jes
Stolz L, Homann G, Winter M, Kasnatscheew J (2021) The Sand equation and its enormous practical relevance for solid-state lithium metal batteries. Mater Today 44:9–14. https://doi.org/10.1016/j.mattod.2020.11.025
Stolz L, Homann G, Winter M, Kasnatscheew J (2021) Kinetical threshold limits in solid-state lithium batteries: data on practical relevance of sand equation. Data Br 34:106688. https://doi.org/10.1016/j.dib.2020.106688
Homann G, Stolz L, Nair J et al (2020) Poly(Ethylene Oxide)-based electrolyte for solid-state-lithium-batteries with high voltage positive electrodes: evaluating the role of electrolyte oxidation in rapid cell failure. Sci Rep 10:2–10. https://doi.org/10.1038/s41598-020-61373-9
Stolz L, Röser S, Homann G et al (2021) Pragmatic approaches to correlate between the physicochemical properties of a linear poly(ethylene oxide)-based solid polymer electrolyte and the performance in a high-voltage li-metal battery. J Phys Chem C 125:18089–18097. https://doi.org/10.1021/acs.jpcc.1c03614
Méry A, Rousselot S, Lepage D, Dollé M (2021) A Critical Review for an Accurate Electrochemical Stability Composite Electrolytes. Materials (Basel)
Stephan AM (2006) Review on gel polymer electrolytes for lithium batteries. Eur Polym J 42:21–42. https://doi.org/10.1016/j.eurpolymj.2005.09.017
Saikia D, Chen-Yang Y, Chen Y, Li SIL Y (2008) Investigation of ionic conductivity of composite gel polymer electrolyte membranes based on P(VDF-HFP), LiClO4 and silica aerogel for lithium ion battery. Desalination 234:24–32
Cheng X, Pan J, Zhao Y et al (2018) Gel polymer electrolytes for electrochemical energy storage. Adv Energy Mater 8:1–16. https://doi.org/10.1002/aenm.201702184
Wang Y, Zhong WH (2015) Development of electrolytes towards achieving safe and high-performance energy-storage devices: A review. ChemElectroChem 2:22–36. https://doi.org/10.1002/celc.201402277
Zhou D, He YB, Cai Q et al (2014) Investigation of cyano resin-based gel polymer electrolyte: in situ gelation mechanism and electrode-electrolyte interfacial fabrication in lithium-ion battery. J Mater Chem A 2:20059–20066. https://doi.org/10.1039/c4ta04504a
Zhou D, Shanmukaraj D, Tkacheva A et al (2019) Polymer electrolytes for lithium-based batteries: advances and prospects. Chem 5:2326–2352. https://doi.org/10.1016/j.chempr.2019.05.009
Hoang Huy VP, So S, Hur J (2021) Inorganic fillers in composite gel polymer electrolytes for high-performance lithium and non-lithium polymer batteries. Nanomaterials 11(3):614. https://doi.org/10.3390/nano11030614 . Open access Journal
Ngai KS, Ramesh S, Ramesh K, Juan JC (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics (Kiel) 22:1259–1279. https://doi.org/10.1007/s11581-016-1756-4
Yao P, Yu H, Ding Z, Liu Y, Lu J, Lavorgna M, ... & Liu X (2019) Review on polymer-based composite electrolytes for lithium batteries. Front Chem 7:522
Florjańczyk Z, Marcinek M, Wieczorek W, Langwald N (2004) Review of PEO based composite polymer electrolytes. Pol J Chem 78:1279–1304
Abraham KM (1993) Directions in secondary lithium battery research and development. Electrochim Acta 38:1233–1248
Bruce PG (1995) Structure and electrochemistry of polymer electrolytes. Electrochim Acta 40:2077–2085. https://doi.org/10.1016/0013-4686(95)00144-4
Gadjourova Z, Andreev YG, Tunstall DP, Bruce PG (2001) Ionic conductivity in crystalline polymer electrolytes. Nature 412:520–523. https://doi.org/10.1038/35087538
Lightfoot P, Mehta MA, Bruce PG (1993) Crystal Structure of the Polymer Electrolyte Poly ( ethylene oxide ) $ _ 3 $ : LiCF $ _ 3 $ SO $ _ 3 $ Author ( s ): P . Lightfoot , M . A . Mehta and P . G . Bruce Published by : American Association for the Advancement of Science Stable URL : https://www.Science (80- ) 262:883–885
Croce F, Curini R, Martinelli A et al (1999) Physical and chemical properties of nanocomposite polymer electrolytes. J Phys Chem B 103:10632–10638. https://doi.org/10.1021/jp992307u
Pedersen CJ (1978) Synthetic multidentate macrocyclic compounds. ACADEMIC PRESS, INC.
Deng F, Wang X, He D et al (2015) Microporous polymer electrolyte based on PVDF/PEO star polymer blends for lithium ion batteries. J Memb Sci 491:82–89. https://doi.org/10.1016/j.memsci.2015.05.021
Polu AR, Rhee HW (2015) Nanocomposite solid polymer electrolytes based on poly(ethylene oxide)/POSS-PEG (n=13.3) hybrid nanoparticles for lithium ion batteries. J Ind Eng Chem 31:323–329. https://doi.org/10.1016/j.jiec.2015.07.005
H. Aydın AB, (2012) Synthesis, characterization, and ionic conductivity of novel crosslinked polymer electrolytes for Li-ion batteries. J Appl Polym Sci 124:1193–1199
YL, Ni’Mah , Cheng MY, Cheng JH, et al (2015) Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J Power Sources 278:375–381. https://doi.org/10.1016/j.jpowsour.2014.11.047
Rolland J, Brassinne J, Bourgeois JP et al (2014) Chemically anchored liquid-PEO based block copolymer electrolytes for solid-state lithium-ion batteries. J Mater Chem A 2:11839–11846. https://doi.org/10.1039/c4ta02327g
Ito Y, Kanehori K, Miyauchi K, Kudo T (1987) Ionic conductivity of electrolytes formed from PEO-LiCF3SO3 complex low molecular weight poly(ethylene glycol). J Mater Sci 22:1845–1849. https://doi.org/10.1007/BF01132415
Polu AR, Rhee HW (2017) Ionic liquid doped PEO-based solid polymer electrolytes for lithium-ion polymer batteries. Int J Hydrogen Energy 42:7212–7219. https://doi.org/10.1016/j.ijhydene.2016.04.160
Zhu L, Zhu P, Fang Q et al (2018) A novel solid PEO/LLTO-nanowires polymer composite electrolyte for solid-state lithium-ion battery. Electrochim Acta 292:718–726. https://doi.org/10.1016/j.electacta.2018.10.005
Zhu P, Yan C, Dirican M et al (2018) Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. J Mater Chem A 6:4279–4285. https://doi.org/10.1039/c7ta10517g
Chen F, Yang D, Zha W et al (2017) Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries. Electrochim Acta 258:1106–1114. https://doi.org/10.1016/j.electacta.2017.11.164
Wang Q, Liu X, Cui Z et al (2020) A fluorinated polycarbonate based all solid state polymer electrolyte for lithium metal batteries. Electrochim Acta 337:135843. https://doi.org/10.1016/j.electacta.2020.135843
Alamgir M, Abraham KM (1993) Li Ion Conductive Electrolytes Based on Poly(vinyl chloride). J Electrochem Soc 140:L96–L97. https://doi.org/10.1149/1.2221654
Tsutsumi H, Doi S, Onimura K, Oishi T (2002) Conductivity enhancement of poly(vinylimidazoline)-based electrolytes by addition of cascade nitrile. Electrochemistry 70:94–98. https://doi.org/10.5796/electrochemistry.70.94
Gopalan AI, Santhosh P, Manesh KM et al (2008) Development of electrospun PVdF-PAN membrane-based polymer electrolytes for lithium batteries. J Memb Sci 325:683–690. https://doi.org/10.1016/j.memsci.2008.08.047
Watanabe M, Kanba M, Nagaoka K, Shinohara I (1982) Ionic conductivity of hybrid films based on polyacrylonitrile and their battery application. J Appl Polym Sci 27:4191–4198. https://doi.org/10.1002/app.1982.070271110
Watanabe M, Kanba M, Nagaoka K, Shinohara I (1983) ionic conductivity of hybrid films composed of polyacrylonitrile, ethylene carbonate, and LiClo4. J Polym Sci Part A-2 Polym Phys 21:939–948. https://doi.org/10.1002/pol.1983.180210610
Appetecchi GB, Croce F, Romagnoli P et al (1999) High-performance gel-type lithium electrolyte membranes. Electrochem commun 1:83–86. https://doi.org/10.1016/S1388-2481(99)00011-9
Appetecchi GB, Croce F, Scrosati B (1995) Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes. Electrochim Acta 40:991–997. https://doi.org/10.1016/0013-4686(94)00345-2
Choi BK, Kim YW, Shin HK (2000) Ionic conduction in PEO-PAN blend polymer electrolytes. Electrochim Acta 45:1371–1374. https://doi.org/10.1016/S0013-4686(99)00345-X
Zhang X, Xu BQ, Lin YH et al (2018) Effects of Li6.75La3Zr1.75Ta0.25O12 on chemical and electrochemical properties of polyacrylonitrile-based solid electrolytes. Solid State Ionics 327:32–38. https://doi.org/10.1016/j.ssi.2018.10.023
Wang Q, Zhang H, Cui Z et al (2019) Siloxane-based polymer electrolytes for solid-state lithium batteries. Energy Storage Mater 23:466–490. https://doi.org/10.1016/j.ensm.2019.04.016
Nam-Soon C, Jung-Ki P (2001) New polymer electrolytes based on PVC/PMMA blend for plastic lithium-ion batteries. Electrochim Acta 46:1453–1459
Rajendran S, Mahendran O, Kannan R (2002) Ionic conductivity studies in composite solid polymer electrolytes based on methylmethacrylate. J Phys Chem Solids 63:303–307. https://doi.org/10.1016/S0022-3697(01)00144-5
IIJIMA TAKASHI, TOYOGUCHI YOSHINORI EN, 1985 Quasi-solid organic electrolytes gelatinized with polymethylmethacrylate and their applications for lithium batteries Denki Kagaku 53 619 623
Bohnke O, Frand G, Rezrazi M et al (1993) Fast ion transport in new lithium electrolytes gelled with PMMA. 2. Influence of lithium salt concentration. Solid State Ionics 66:105–112. https://doi.org/10.1016/0167-2738(93)90033-Y
Rhoo HJ, Kim HT, Park JK, Hwang TS (1997) Ionic conduction in plasticized PVC/PMMA blend polymer electrolytes. Electrochim Acta 42:1571–1579. https://doi.org/10.1016/S0013-4686(96)00318-0
Ramesh S, Liew CW (2013) Dielectric and FTIR studies on blending of [xPMMA-(1–X)PVC] with LiTFSI. Meas J Int Meas Confed 46:1650–1656. https://doi.org/10.1016/j.measurement.2013.01.003
Ramesh S, Lu SC (2008) Effect of nanosized silica in poly(methyl methacrylate)-lithium bis(trifluoromethanesulfonyl)imide based polymer electrolytes. J Power Sources 185:1439–1443. https://doi.org/10.1016/j.jpowsour.2008.07.055
Liu LL, Li ZH, Xia QL et al (2012) Electrochemical study of P(VDF-HFP)/PMMA blended polymer electrolyte with high-temperature stability for polymer lithium secondary batteries. Ionics (Kiel) 18:275–281. https://doi.org/10.1007/s11581-011-0632-5
Sun J, Li Y, Zhang Q, et al (2019) A highly ionic conductive poly(methyl methacrylate) composite electrolyte with garnet-typed Li6.75La3Zr1.75Nb0.25O12 nanowires. Chem Eng J 375:121922. https://doi.org/10.1016/j.cej.2019.121922
Liu B, Huang Y, Zhao L et al (2018) A novel non-woven fabric supported gel polymer electrolyte based on poly(methylmethacrylate-polyhedral oligomeric silsesquioxane) by phase inversion method for lithium ion batteries. J Memb Sci 564:62–72. https://doi.org/10.1016/j.memsci.2018.07.014
Sivaraj P, Abhilash KP, Nalini B et al (2020) Performance enhancement of PVDF/LiCIO4 based nanocomposite solid polymer electrolytes via incorporation of Li0.5La0.5TiO3 nano filler for all-solid-state batteries. Macromol Res 28:739–750. https://doi.org/10.1007/s13233-020-8096-y
Wang F, Li L, Yang X et al (2018) Influence of additives in a PVDF-based solid polymer electrolyte on conductivity and Li-ion battery performance. Sustain Energy Fuels 2:492–498. https://doi.org/10.1039/c7se00441a
Watanabe M, Kanba M, Matsuda H, Tsunemi K, Mizoguchi K, Eishun Tsuchida IS (1981) High lithium ionic conductivity of polymeric solid electrolytes. Macromol Rapid Commun 2:741–744
Tsuchida E, Ohno H, Tsunemi K (1983) Conduction of lithium ions in polyvinylidene fluoride and its derivatives-I. Electrochim Acta 28:591–595. https://doi.org/10.1016/0013-4686(83)85049-X
Choe HS, Giaccai J, Alamgir M, Abraham KM (1995) Preparation and characterization of poly(vinyl sulfone)- and poly(vinylidene fluoride)-based electrolytes. Electrochim Acta 40:2289–2293. https://doi.org/10.1016/0013-4686(95)00180-M
Janakiraman, S. Abhijith Surendran SG, S. Anandhan, Venimadhav A (2018) A new strategy of PVDF based Li-salt polymer electrolyte through electrospinning for lithium battery application. Mater Res express 6:035303
Xie M, Li L, Zhang Y et al (2020) Mastering high ion conducting of room-temperature all-solid-state lithium-ion batteries via safe phthaloyl starch-poly(vinylidene fluoride)–based polymer electrolyte. Ionics (Kiel) 26:1109–1117. https://doi.org/10.1007/s11581-019-03309-6
Bose P, Deb D, Bhattacharya S (2019) Lithium-polymer battery with ionic liquid tethered nanoparticles incorporated P(VDF-HFP) nanocomposite gel polymer electrolyte. Electrochim Acta 319:753–765. https://doi.org/10.1016/j.electacta.2019.07.013
Jia W, Li Z, Wu Z et al (2018) Graphene oxide as a filler to improve the performance of PAN-LiClO4 flexible solid polymer electrolyte. Solid State Ionics 315:7–13. https://doi.org/10.1016/j.ssi.2017.11.026
Ramesh S, Soon-Chien L (2012) Enhancement of ionic conductivity and structural properties by 1-Butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid in poly(vinylidene fluoride–hexafluoropropylene)-based polymer electrolytes. Appl Polym Sci 126:484–492
Kim KM, Ryu KS, Kang S-G et al (2001) The effect of silica addition on the properties of poly((vinylidene fluoride)-co-hexafluoropropylene)-based polymer electrolytes. Macromol Chem Phys 202:866–872. https://doi.org/10.1002/1521-3935(20010301)202:6%3c866::aid-macp866%3e3.3.co;2-3
Costa CM, Silva MM, Lanceros-Méndez S (2013) Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv 3:11404–11417. https://doi.org/10.1039/c3ra40732b
Abraham KM, Jiang Z, Carroll B (1997) Highly Conductive PEO-like polymer electrolytes. Chem Mater 9:1978–1988. https://doi.org/10.1021/cm970075a
Saikia D, Kumar A (2004) Ionic conduction in P(VDF-HFP)/PVDF-(PC + DEC)-LiClO4 polymer gel electrolytes. Electrochim Acta 49:2581–2589. https://doi.org/10.1016/j.electacta.2004.01.029
Gonçalves R, Miranda D, Almeida AM et al (2019) Solid polymer electrolytes based on lithium bis(trifluoromethanesulfonyl)imide/poly(vinylidene fluoride -co-hexafluoropropylene) for safer rechargeable lithium-ion batteries. Sustain Mater Technol 21:1–31. https://doi.org/10.1016/j.susmat.2019.e00104
Liang YF, Deng SJ, Xia Y et al (2018) A superior composite gel polymer electrolyte of Li7La3Zr2O12- poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) for rechargeable solid-state lithium ion batteries. Mater Res Bull 102:412–417. https://doi.org/10.1016/j.materresbull.2018.02.051
Ramesh S, Yin TS, Liew CW (2011) Effect of dibutyl phthalate as plasticizer on high-molecular weight poly(vinyl chloride)-lithium tetraborate-based solid polymer electrolytes. Ionics (Kiel) 17:705–713. https://doi.org/10.1007/s11581-011-0568-9
Sukeshini AM, Nishimoto A, Watanabe M (1996) Transport and electrochemical characterization of plasticized poly(vinyl chloride) solid electrolytes. Solid State Ionics 86–88:385–393. https://doi.org/10.1016/0167-2738(96)00156-7
Manuel Stephan A, Thirunakaran R, Renganathan NG et al (1999) A study on polymer blend electrolyte based on PVC/PMMA with lithium salt. J Power Sources 81–82:752–758. https://doi.org/10.1016/s0378-7753(99)00148-2
Stephan AM, Renganathan NG, Kumar TP et al (2000) Ionic conductivity studies on plasticized PVC/PMMA blend polymer electrolyte containing LiBF4 and LiCF3SO3. Solid State Ionics 130:123–132. https://doi.org/10.1016/S0167-2738(00)00440-9
Stephan AM, Kumar TP, Renganathan NG et al (2000) Ionic conductivity and FT-IR studies on plasticized PVC/PMMA blend polymer electrolytes. J Power Sources 89:80–87. https://doi.org/10.1016/S0378-7753(00)00379-7
Sung H, Wang Y, Wan C (1998) Preparation and characterization of poly(vinyl chloride-co-vinyl acetate)-based gel electrolytes for Li-Ion batteries. J Electrochem Soc 145:1207–1211. https://doi.org/10.1149/1.1838440
Jagadeesan A, Sasikumar M, Jeevani R et al (2019) Fabrication of BaTiO3 ceramic filler incorporated PVC-PEMA based blend nanocomposite gel polymer electrolytes for Li ion battery applications. J Mater Sci Mater Electron 30:17181–17194. https://doi.org/10.1007/s10854-019-02065-7
A Jagadeesan, M Sasikumar, R Hari Krishna, N Raja, D Gopalakrishna SV and PS (2019) High electrochemical performance of nano TiO2 ceramic filler incorporated PVC-PEMA composite gel polymer electrolyte for Li-ion battery applications. Mater Res express 6:105524
Dasenbrock CO, Ridgway TH, Seliskar CJ, Heineman WR (1998) Evaluation of the electrochemical characteristics of a poly(vinyl alcohol)/poly(acrylic acid) polymer blend. Electrochim Acta 43:3497–3502. https://doi.org/10.1016/S0013-4686(98)00097-8
Jen MY, Hung ZW, Chun CY (2008) Modification and characterization of semi-crystalline poly(vinyl alcohol) with interpenetrating poly(acrylic acid) by UV radiation method for alkaline solid polymer electrolytes membrane. J Memb Sci 322:74–80. https://doi.org/10.1016/j.memsci.2008.05.035
Yang CC, Wu GM (2009) Study of microporous PVA/PVC composite polymer membrane and it application to MnO2 capacitors. Mater Chem Phys 114:948–955. https://doi.org/10.1016/j.matchemphys.2008.11.009
Lu Y, Wang D, Li T et al (2009) Poly(vinyl alcohol)/poly(acrylic acid) hydrogel coatings for improving electrode-neural tissue interface. Biomaterials 30:4143–4151. https://doi.org/10.1016/j.biomaterials.2009.04.030
Qiao J, Okada T, Ono H (2009) High molecular weight PVA-modified PVA/PAMPS proton-conducting membranes with increased stability and their application in DMFCs. Solid State Ionics 180:1318–1323. https://doi.org/10.1016/j.ssi.2009.08.010
Guzman-Puyol S, Ceseracciu L, Heredia-Guerrero JA et al (2015) Effect of trifluoroacetic acid on the properties of polyvinyl alcohol and polyvinyl alcohol-cellulose composites. Chem Eng J 277:242–251. https://doi.org/10.1016/j.cej.2015.04.092
Hirankumar G, Selvasekarapandian S, Kuwata N et al (2005) Thermal, electrical and optical studies on the poly(vinyl alcohol) based polymer electrolytes. J Power Sources 144:262–267. https://doi.org/10.1016/j.jpowsour.2004.12.019
Vargas MA, Vargas RA, Mellander BE (1999) New proton conducting membranes based on PVAL/H3PO2/H2O. Electrochim Acta 44:4227–4232. https://doi.org/10.1016/S0013-4686(99)00137-1
Vargas RA, Zapata VH, Matallana E, Vargas MA (2001) More thermal studies on the PVOH/H3PO2/H2O solid proton conductor gels. Electrochim Acta 46:1699–1702. https://doi.org/10.1016/S0013-4686(00)00773-8
Vargas MA, Vargas RA, Mellander BE (2000) Phase behavior of a PVAL-based polymer proton conductor. Phys Status Solidi Basic Res 220:615–624. https://doi.org/10.1002/1521-3951(200007)220:1%3c615::AID-PSSB615%3e3.0.CO;2-T
Vargas MA, Vargas RA, Mallander BE (2000) More studies on the PVA1 + H3PO2 + H2O proton conductor gels. Electrochim Acta 45:1399–1403. https://doi.org/10.1016/S0013-4686(99)00350-3
Rangasamy VS, Thayumanasundaram S, Locquet JP (2019) Solid polymer electrolytes with poly(vinyl alcohol) and piperidinium based ionic liquid for Li-ion batteries. Solid State Ionics 333:76–82. https://doi.org/10.1016/j.ssi.2019.01.024
Wang J, Zhao Z, Song S et al (2018) High performance poly(vinyl alcohol)-based Li-ion conducting gel polymer electrolyte films for electric double-layer capacitors. Polymers (Basel). https://doi.org/10.3390/polym10111179
Sasikumar M, Raja M, Krishna RH et al (2018) Influence of hydrothermally synthesized cubic-structured BaTiO3 ceramic fillers on ionic conductivity, mechanical integrity, and thermal behavior of P(VDF-HFP)/PVAc-based composite solid polymer electrolytes for lithium-ion batteries. J Phys Chem C 122:25741–25752. https://doi.org/10.1021/acs.jpcc.8b03952
Hema M, Tamilselvi P (2016) Lithium ion conducting PVA:PVdF polymer electrolytes doped with nano SiO2 and TiO2 filler. J Phys Chem Solids 96–97:42–48. https://doi.org/10.1016/j.jpcs.2016.04.008
Francis KMG, Subramanian S, Shunmugavel K et al (2016) Lithium ion-conducting blend polymer electrolyte based on PVA–PAN doped with lithium nitrate. Polym–Plast Technol Eng 55:25–35. https://doi.org/10.1080/03602559.2015.1050523
Pareek K, Ghosh A, Sen SK, Banerjee S (2010) Synthesis, characterization and properties of new fluorinated poly(imide siloxane) co-polymers from 4,4′-(hexafluoro-isopropylidene)diphthalic anhydride. Des Monomers Polym 13:221–236. https://doi.org/10.1163/138577210X12634696333550
Zhang ZC, Jin JJ, Bautista F et al (2004) Ion conductive characteristics of cross-linked network polysiloxane-based solid polymer electrolytes. Solid State Ionics 170:233–238. https://doi.org/10.1016/j.ssi.2004.04.007
Liu M, Jin B, Zhang Q et al (2018) High-performance solid polymer electrolytes for lithium ion batteries based on sulfobetaine zwitterion and poly (ethylene oxide) modified polysiloxane. J Alloys Compd 742:619–628. https://doi.org/10.1016/j.jallcom.2018.01.263
Ratner. B.D, Hoffman A.S, Schoen F.J LJ. (2004) Biomaterials science: An introduction to materials in medicine. Elsevier
Walkowiak M, Schroeder G, Gierczyk B et al (2007) New lithium ion conducting polymer electrolytes based on polysiloxane grafted with Si-tripodand centers. Electrochem commun 9:1558–1562. https://doi.org/10.1016/j.elecom.2007.02.019
Megahed S, Bruno S (1994) Lithium-ion rechargeable batteries. J Power Sources 51:79–104
Deka JR, Saikia D, Lou GW et al (2019) Design, synthesis and characterization of polysiloxane and polyetherdiamine based comb-shaped hybrid solid polymer electrolytes for applications in electrochemical devices. Mater Res Bull 109:72–81. https://doi.org/10.1016/j.materresbull.2018.09.003
Feng J, Zhuo RX, Zhang XZ (2012) Construction of functional aliphatic polycarbonates for biomedical applications. Prog Polym Sci 37:211–236. https://doi.org/10.1016/j.progpolymsci.2011.07.008
Suriano F, Coulembier O, Hedrick JL, Dubois P (2011) Functionalized cyclic carbonates: from synthesis and metal-free catalyzed ring-opening polymerization to applications. Polym Chem 2:528–533. https://doi.org/10.1039/c0py00211a
Zhang X, Fevre M, Jones GO, Waymouth RM (2018) Catalysis as an enabling science for sustainable polymers. Chem Rev 118:839–885. https://doi.org/10.1021/acs.chemrev.7b00329
Hong M, Chen EYX (2017) Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem 19:3692–3706. https://doi.org/10.1039/c7gc01496a
Zhu Y, Romain C, Williams CK (2016) Sustainable polymers from renewable resources. Nature 540:354–362. https://doi.org/10.1038/nature21001
Tempelaar S, Mespouille L, Coulembier O et al (2013) Synthesis and post-polymerisation modifications of aliphatic poly(carbonate)s prepared by ring-opening polymerisation. Chem Soc Rev 42:1312–1336. https://doi.org/10.1039/c2cs35268k
Pratt RC, Nederberg F, Waymouth RM, Hedrick JL (2008) Tagging alcohols with cyclic carbonate: a versatile equivalent of (meth)acrylate for ring-opening polymerization. Chem Commun 2:114–116. https://doi.org/10.1039/b713925j
Mindemark J, Bowden T (2010) Efficient DNA binding and condensation using low molecular weight, low charge density cationic polymer amphiphiles. Macromol Rapid Commun 31:1378–1382. https://doi.org/10.1002/marc.201000141
Mindemark J, Bowden T (2011) Synthesis and polymerization of alkyl halide-functional cyclic carbonates. Polymer (Guildf) 52:5716–5722. https://doi.org/10.1016/j.polymer.2011.10.027
Mindemark J, Bowden T (2012) Diversity in cyclic carbonates: synthesis of triazole-functional monomers using click chemistry. Polym Chem 3:1399–1401. https://doi.org/10.1039/c2py20152f
Sugimoto H, Inoue S (2004) Copolymerization of carbon dioxide and epoxide. J Polym Sci Part A Polym Chem 42:5561–5573. https://doi.org/10.1002/pola.20319
Lu XB, Darensbourg DJ (2012) Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem Soc Rev 41:1462–1484. https://doi.org/10.1039/c1cs15142h
Sun B, Mindemark J, Edström K, Brandell D (2014) Polycarbonate-based solid polymer electrolytes for Li-ion batteries. Solid State Ionics 262:738–742. https://doi.org/10.1016/j.ssi.2013.08.014
Zhang J, Zang X, Wen H et al (2017) High-voltage and free-standing poly(propylene carbonate)/Li6.75La3Zr1.75Ta0.25O12 composite solid electrolyte for wide temperature range and flexible solid lithium ion battery. J Mater Chem A 5:4940–4948. https://doi.org/10.1039/c6ta10066j
Zhao J, Zhang J, Hu P et al (2016) A sustainable and rigid-flexible coupling cellulose-supported poly(propylene carbonate) polymer electrolyte towards 5 v high voltage lithium batteries. Electrochim Acta 188:23–30. https://doi.org/10.1016/j.electacta.2015.11.088
Zhan X, Zhang J, Liu M et al (2019) Advanced polymer electrolyte with enhanced electrochemical performance for lithium-ion batteries: effect of nitrile-functionalized ionic liquid. ACS Appl Energy Mater 2:1685–1694. https://doi.org/10.1021/acsaem.8b01733
Chai J, Liu Z, Ma J et al (2017) In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv Sci 4:1–9. https://doi.org/10.1002/advs.201600377
Mindemark J, Sun B, Törmä E, Brandell D (2015) High-performance solid polymer electrolytes for lithium batteries operational at ambient temperature. J Power Sources 298:166–170. https://doi.org/10.1016/j.jpowsour.2015.08.035
Amass W, Amass A, Tighe B (1998) A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int 47:89–144. https://doi.org/10.1002/(SICI)1097-0126(1998100)47:2%3c89::AID-PI86%3e3.0.CO;2-F
Coombes AGA, Rizzi SC, Williamson M et al (2004) Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 25:315–325. https://doi.org/10.1016/S0142-9612(03)00535-0
Ramesh S, Uma O, Shanti R et al (2014) Preparation and characterization of poly (ethyl methacrylate) based polymer electrolytes doped with 1-butyl-3-methylimidazolium trifluoromethanesulfonate. Meas J Int Meas Confed 48:263–273. https://doi.org/10.1016/j.measurement.2013.11.025
Kam W, Liew CW, Lim JY, Ramesh S (2014) Electrical, structural, and thermal studies of antimony trioxide-doped poly(acrylic acid)-based composite polymer electrolytes. Ionics (Kiel) 20:665–674. https://doi.org/10.1007/s11581-013-1012-0
Mauger A, Julien CM, Paolella A et al (2018) A comprehensive review of lithium salts and beyond for rechargeable batteries: progress and perspectives. Mater Sci Eng R Reports 134:1–21. https://doi.org/10.1016/j.mser.2018.07.001
Aravindan V, Gnanaraj J, Madhavi S, Liu HK (2011) Lithium-ion conducting electrolyte salts for lithium batteries. Chem–A Eur J 17:14326–14346. https://doi.org/10.1002/chem.201101486
Marcinek M, Syzdek J, Marczewski M et al (2015) Electrolytes for Li-ion transport–Review. Solid State Ionics 276:107–126. https://doi.org/10.1016/j.ssi.2015.02.006
Younesi R, Veith GM, Johansson P, Edström K, Vegge T (2015) Lithium salts for advanced lithium batteries: Li–metal, Li–O 2, and Li–S. Energy Environ Sci 8(7):1905–1922
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603. https://doi.org/10.1021/cm901452z
Walker CW, Cox JD, Salomon M (1996) Conductivity and electrochemical stability of electrolytes containing organic solvent mixtures with Lithium tris(Trifluoromethanesulfonyl)methide. J Electrochem Soc 143:L80–L82. https://doi.org/10.1149/1.1836607
Kanamura K, Okagawa T, Takeharaichiro Z (1995) Electrochemical oxidation of propylene carbonate (containing various salts) on aluminium electrodes. J Power Sources 57:119–123. https://doi.org/10.1016/0378-7753(95)02265-1
Ue M, Murakami A, Nakamura S (2002) Anodic stability of several anions examined by ab initio molecular orbital and density functional theories. J Electrochem Soc 149:A1572. https://doi.org/10.1149/1.1517579
Zinigrad E, Larush-Asraf L, Gnanaraj JS et al (2005) On the thermal stability of LiPF6. Thermochim Acta 438:184–191. https://doi.org/10.1016/j.tca.2005.09.006
Thermal Stability of LiPF 6 Salt and Li-ion Battery Electrolytes Containing LiPF 6 Hui Yang
Stenzel YP, Horsthemke F, Winter M, Nowak S (2019) Chromatographic techniques in the research area of lithium ion batteries: current state-of-the-art. Separations 6:17–23. https://doi.org/10.3390/separations6020026
Campion CL, Li W, Euler WB et al (2004) Suppression of toxic compounds produced in the decomposition of lithium-ion battery electrolytes. Electrochem Solid-State Lett 7:194–197. https://doi.org/10.1149/1.1738551
Tasaki K, Kanda K, Nakamura S, Ue M (2003) Decomposition of LiPF[sub 6] and stability of PF[sub 5] in li-ion battery electrolytes. J Electrochem Soc 150:A1628. https://doi.org/10.1149/1.1622406
Campion CL, Li W, Lucht BL (2005) Thermal decomposition of LiPF[sub 6]-based electrolytes for lithium-ion batteries. J Electrochem Soc 152:A2327. https://doi.org/10.1149/1.2083267
Gnanaraj JS, Zinigrad E, Asraf L et al (2003) A detailed investigation of the thermal reactions of LiPF[sub 6] solution in organic carbonates using ARC and DSC. J Electrochem Soc 150:A1533. https://doi.org/10.1149/1.1617301
Heider U, Oesten R, Jungnitz M (1999) Challenge in manufacturing electrolyte solutions for lithium and lithium ion batteries quality control and minimizing contamination level. J Power Sources 81–82:119–122. https://doi.org/10.1016/S0378-7753(99)00142-1
Plakhotnyk AV, Ernst L, Schmutzler R (2005) Hydrolysis in the system LiPF 6–propylene carbonate–dimethyl carbonate–H 2O. J Fluor Chem 126:27–31. https://doi.org/10.1016/j.jfluchem.2004.09.027
Andersson AM, Herstedt M, Bishop AG, Edström K (2002) The influence of lithium salt on the interfacial reactions controlling the thermal stability of graphite anodes. Electrochim Acta 47:1885–1898. https://doi.org/10.1016/S0013-4686(02)00044-0
Zhang SS, Xu K, Jow TR (2002) Study of LiBF[sub 4] as an electrolyte salt for a Li-Ion battery. J Electrochem Soc 149:A586. https://doi.org/10.1149/1.1466857
Takami N, Ohsaki T, Hasebe H, Yamamoto M (2002) Laminated thin Li-Ion batteries using a liquid electrolyte. J Electrochem Soc 149:A9. https://doi.org/10.1149/1.1420704
Zhang SS, Xu K, Jow TR (2003) Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte. J Solid State Electrochem 7:147–151. https://doi.org/10.1007/s10008-002-0300-9
Ue M, Fujii T, Bin ZZ et al (2006) Electrochemical properties of Li[CnF2n+1BF 3] as electrolyte salts for lithium-ion cells. Solid State Ionics 177:323–331. https://doi.org/10.1016/j.ssi.2005.10.023
Webber A (1991) Conductivity and viscosity of Solutions of LiCF3 SO 3, Li (CF 3 SO 2) 2 N, and their mixtures. J Electrochem Soc 138:2586–2590. https://doi.org/10.1149/1.2087287
Aurbach D, Markovsky B, Levi MD et al (1999) New insights into the interactions between electrode materials and electrolyte solutions for advanced nonaqueous batteries. J Power Sources 81–82:95–111. https://doi.org/10.1016/S0378-7753(99)00187-1
Arai J, Pe L (1999) Anodic dissolution of aluminium in organic electrolytes containing per ¯ uoroalkylsulfonyl imides. J Appl Electrochem 29:1053–1061
Krause LJ, Lamanna W, Summerfield J et al (1997) Corrosion of aluminum at high voltages in non-aqueous electrolytes containing perfluoroalkylsulfonyl imides; new lithium salts for lithium-ion cells. J Power Sources 68:320–325. https://doi.org/10.1016/S0378-7753(97)02517-2
Brox S, Röser S, Streipert B et al (2017) Innovative, non-corrosive LiTFSI cyanoester-based electrolyte for safer 4 V lithium-Ion batteries. ChemElectroChem 4:304–309. https://doi.org/10.1002/celc.201600610
Xu W, Angell AC (2001) LiBOB and Its derivatives. Electrochem Solid-State Lett 4:3–6
Azeez F, Fedkiw PS (2010) Conductivity of libob-based electrolyte for lithium-ion batteries. J Power Sources 195:7627–7633. https://doi.org/10.1016/j.jpowsour.2010.06.021
Xue ZM, Bin SB, Zhou W, Chen CH (2011) A new lithium salt with dihydroxybenzene and lithium tetrafluoroborate for lithium battery electrolytes. J Power Sources 196:8710–8713. https://doi.org/10.1016/j.jpowsour.2011.06.060
Liu Z, Chai J, Xu G et al (2015) Functional lithium borate salts and their potential application in high performance lithium batteries. Coord Chem Rev 292:56–73. https://doi.org/10.1016/j.ccr.2015.02.011
Zhang X, Devine TM (2006) Passivation of aluminum in lithium-ion battery electrolytes with LiBOB. J Electrochem Soc 153:B365. https://doi.org/10.1149/1.2218269
Xu K (2008) Tailoring Electrolyte Composition for LiBOB. J Electrochem Soc 155:A733. https://doi.org/10.1149/1.2961055
Abraham DP, Furczon MM, Kang SH et al (2008) Effect of electrolyte composition on initial cycling and impedance characteristics of lithium-ion cells. J Power Sources 180:612–620. https://doi.org/10.1016/j.jpowsour.2008.02.047
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195:939–954. https://doi.org/10.1016/j.jpowsour.2009.08.089
Schmidt M, Heider U, Kuehner A et al (2001) Lithium fluoroalkylphosphates: a new class of conducting salts for electrolytes for high energy lithium-ion batteries. J Power Sources 97–98:557–560. https://doi.org/10.1016/S0378-7753(01)00640-1
Koch VR, Goldman JL, Mattos CJ, Mulvaney M (1982) Specular lithium deposits from lithium hexafluoroarsenate/diethyl ether electrolytes. J Electrochem Soc 129:1–4. https://doi.org/10.1149/1.2123756
Aurbach D, Markovsky B, Shechter A et al (1996) A comparative study of synthetic graphite and li electrodes in electrolyte solutions based on ethylene carbonate-dimethyl carbonate mixtures. J Electrochem Soc 143:3809–3820. https://doi.org/10.1149/1.1837300
Aurbach D, Ein-Eli Y, Markovsky B et al (1995) The study of electrolyte solutions based on ethylene and diethyl carbonates for rechargeable li batteries: II. Graphite Electrodes J Electrochem Soc 142:2882–2890. https://doi.org/10.1149/1.2048659
Yoshimatsu I, Hirai T, Yamaki J (1988) Lithium electrode morphology during cycling in lithium cells. J Electrochem Soc 135:2422–2427. https://doi.org/10.1149/1.2095351
Plichta E, Slane S, Uchiyama M et al (1989) An improved Li/Li x CoO2 rechargeable Cell. J Electrochem Soc 136:1865–1869. https://doi.org/10.1149/1.2097063
Bushkova OV, Yaroslavtseva TV, Dobrovolsky YA (2017) New lithium salts in electrolytes for lithium-ion batteries (review). Russ J Electrochem 53:677–699. https://doi.org/10.1134/S1023193517070035
Nanjundiah C, Goldman JL, Dominey LA, Koch VR (1988) Electrochemical stability of LiMF6 ( M = P, As, Sb ) in tetrahydrofuran and sulfolane. J Electrochem Soc 135:2914–2917. https://doi.org/10.1149/1.2095462
Chen HP, Fergus JW, Jang BZ (2000) The effect of ethylene carbonate and salt concentration on the conductivity of propylene carbonate∣lithium perchlorate electrolytes. J Electrochem Soc 147:399. https://doi.org/10.1149/1.1393209
Herr R (1990) Organic electrolytes for lithium cells. Electrochim Acta 35:1257–1265. https://doi.org/10.1016/0013-4686(90)90059-9
Newman GH, Francis RW, Gaines LH, Rao BML (1980) Hazard investigations of LiClO4/Dioxolane electrolyte. J Electrochem Soc 127:2025–2027. https://doi.org/10.1149/1.2130056
Jiang G, Maeda S, Yang H et al (2005) All solid-state lithium-polymer battery using poly(urethane acrylate)/nano-SiO2 composite electrolytes. J Power Sources 141:143–148. https://doi.org/10.1016/j.jpowsour.2004.09.004
Croce F, Persi L, Ronci F, Scrosati B (2000) Nanocomposite polymer electrolytes and their impact on the lithium battery technology. Solid State Ionics 135:47–52. https://doi.org/10.1016/S0167-2738(00)00329-5
Jung S, Kim DW, Lee SD et al (2009) Fillers for solid-state polymer electrolytes: highlight. Bull Korean Chem Soc 30:2355–2361. https://doi.org/10.5012/bkcs.2009.30.10.2355
Scrosati B, Croce F, Panero S (2001) Progress in lithium polymer battery R&D. J Power Sources 100:93–100. https://doi.org/10.1016/S0378-7753(01)00886-2
Rajendran S, Uma T (2000) Effect of ceramic oxide on PVC-PMMA hybrid polymer electrolytes. Ionics (Kiel) 6:288–293. https://doi.org/10.1007/BF02374079
Lakshman Dissanayake MAK (2004) Nano-composite solid polymer electrolytes for solid state ionic devices. Ionics (Kiel) 10:221–225. https://doi.org/10.1007/BF02382820
Krawiec W, Scanlon LG, Fellner JP et al (1995) Polymer nanocomposites: a new strategy for synthesizing solid electrolytes for rechargeable lithium batteries. J Power Sources 54:310–315. https://doi.org/10.1016/0378-7753(94)02090-P
Miao R, Liu B, Zhu Z et al (2008) PVDF-HFP-based porous polymer electrolyte membranes for lithium-ion batteries. J Power Sources 184:420–426. https://doi.org/10.1016/j.jpowsour.2008.03.045
Stephan AM, Nahm KS, Anbu Kulandainathan M et al (2006) Poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) based composite electrolytes for lithium batteries. Eur Polym J 42:1728–1734. https://doi.org/10.1016/j.eurpolymj.2006.02.006
Arya A, Sharma AL (2017) Polymer electrolytes for lithium ion batteries: a critical study. Ionics 23(3):497–540
Croce F, Sacchetti S, Scrosati B (2006) Advanced, lithium batteries based on high-performance composite polymer electrolytes. J Power Sources 162:685–689. https://doi.org/10.1016/j.jpowsour.2006.07.038
Yadav GD, Sengupta S (2002) Friedel-Crafts alkylation of diphenyl oxide with benzyl chloride over sulphated zirconia. Org Process Res Dev 6:256–262. https://doi.org/10.1021/op990099y
Croce F, Settimi L, Scrosati B (2006) Superacid ZrO2-added, composite polymer electrolytes with improved transport properties. Electrochem commun 8:364–368. https://doi.org/10.1016/j.elecom.2005.12.002
Occelli ML, Tindwa RM (1983) Physicochemical properties of montmorillonite interlayered with cationic oxyaluminum pillars. Clays Clay Miner 31:22–28. https://doi.org/10.1346/CCMN.1983.0310104
Appetecchi GB, Passerini S (2000) PEO-carbon composite lithium polymer electrolyte. Electrochim Acta 45:2139–2145. https://doi.org/10.1016/S0013-4686(99)00437-5
Izadi HGA (2012) Distribution and stability of carbon nanotubes during multi-pass friction stir processing of carbon nanotube/aluminum composites. Carbon N Y 50:4744–4749
Xi J, Qiu X, Ma X et al (2005) Composite polymer electrolyte doped with mesoporous silica SBA-15 for lithium polymer battery. Solid State Ionics 176:1249–1260. https://doi.org/10.1016/j.ssi.2005.02.016
Xi J, Miao S, Tang X (2004) Selective transporting of lithium ion by shape selective molecular sieves ZSM-5 in PEO-based composite polymer electrolyte. Macromolecules 37:8592–8598. https://doi.org/10.1021/ma048849d
Mishra HK, Parida KM (2002) Studies on sulphated zirconia: Synthesis, physico-chemical characterisation and n-butane isomerisation activity. Appl Catal A Gen 224:179–189. https://doi.org/10.1016/S0926-860X(01)00822-5
Dudley JT, Wilkinson DP, Thomas G et al (1991) Conductivity of electrolytes for rechargeable lithium batteries. J Power Sources 35:59–82. https://doi.org/10.1016/0378-7753(91)80004-H
Capuano F, Croce F, Scrosati B (1991) Composite polymer electrolytes. J Electrochem Soc 138:1918–1922. https://doi.org/10.1149/1.2085900
Linert W, Camard A, Armand M, Michot C (2002) Anions of low Lewis basicity for ionic solid state electrolytes, Linert et al, Coordination chemistry reviews, 2002.pdf. Coord Chem Rev 226:137–141
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sashmitha, K., Rani, M.U. A comprehensive review of polymer electrolyte for lithium-ion battery. Polym. Bull. 80, 89–135 (2023). https://doi.org/10.1007/s00289-021-04008-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-04008-x