Abstract
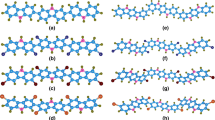
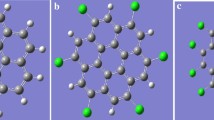
We have investigated the structures, electronic properties, hole and electron mobilities of perfluorinated, perchlorinated and percyanated coronene molecules, using the density functional theory (DFT) and Time Dependent DFT (TDDFT) at the B3LYP-D3/6-311++G(d,p) and \(\omega\)B97XD/6-311++G(d,p) levels and Markus-Hush charge transfer theory. The calculated geometric parameters for coronene and perchlorocoronene are in good agreement with the experimental data. Our theoretical investigations have shown that B3LYP-D3 functional is suitable to well define vibrational assignments for studied molecules. The quantified effect of the complete substitution of peripheral hydrogen atoms with cyanide groups for the key properties relevant for optoelectronic and photonics such as electron affinities, ionization energies, HOMO-LUMO energies, reorganisation energies, optical absorption spectra, and electron mobilities were discussed. Compared to perfluorination and perchlorination, the percyanation of coronene considerably increases the adiabatic/vertical electron affinities (AEAs/VEAs), the electron mobilities, the HOMO-LUMO gap and reduces the LUMO energy level thus indicating an ambipolar behavior and air-stable material. We have discussed the possible implications of cyanide groups as important substitutes for the design of the new organic compounds useful in electronics.






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and it supplementary information file).
References
Sahoo SR, Sharma S, Sahu S (2020) A computational study of anisotropic charge transport in air-stable fluorinated benzobisbenzothiophene (FBBBT) derivatives. J Mole Model 26(1):1. https://doi.org/10.1007/s00894-019-4251-9
Sanyal S, Manna AK, Pati SK (2013) Effect of imide functionalization on the electronic, optical, and charge transport properties of coronene: a theoretical study. J Phys Chem C 117(2):825. https://doi.org/10.1021/jp310362c
Moon H, Zeis R, Borkent EJ, Besnard C, Lovinger AJ, Siegrist T, Kloc C, Bao Z (2004) Synthesis, crystal structure, and transistor performance of tetracene derivatives. J Am Chem Soc 126(47):15322. https://doi.org/10.1021/ja045208p
Wen SH, Li A, Song J, Deng WQ, Han KL, Goddard WA (2009) First-principles investigation of anistropic hole mobilities in organic semiconductors. J Phys Chem B 113(26):8813. https://doi.org/10.1021/jp900512s
Ji LF, Fan JX, Zhang SF, Ren AM (2017) Theoretical investigations into the charge transfer properties of thiophene \(\alpha \)-substituted naphthodithiophene diimides: excellent n-channel and ambipolar organic semiconductors. Phys Chem Chem Phys 19(21):13978. https://doi.org/10.1039/c7cp01114h
Chua LL, Zaumseil J, Chang JF, Ou EC, Ho PK, Sirringhaus H, Friend RH (2005) General observation of n-type field-effect behaviour in organic semiconductors. Nature 434(7030):194. https://doi.org/10.1038/nature03376
Yin J, Chaitanya K, Ju XH (2016) Bromination and cyanation for improving electron transport performance of anthra-tetrathiophene. J Mater Res 31(3):337. https://doi.org/10.1557/jmr.2016.8
Hutchison GR, Ratner MA, Marks TJ (2005) Hopping transport in conductive heterocyclic oligomers: reorganization energies and substituent effects. J Am Chem Soc 127(7):2339. https://doi.org/10.1021/ja0461421
Geng H, Niu Y, Peng Q, Shuai Z, Coropceanu V, Brédas JL (2011) Theoretical study of substitution effects on molecular reorganization energy in organic semiconductors. J Chem Phys 135(10):1. https://doi.org/10.1063/1.3632105
Fawcett J, Trotter J (2014) The crystal and molecular structure of Coronene. Proceed Royal Soc London. Series A. Math Phys Sci 289(1418):366. https://doi.org/10.1098/rspa.1966.0017
Ejuh GW, Samuel N, Fridolin TN, Marie NJ (2016) Computational determination of the electronic and nonlinear optical properties of the molecules 2-(4-aminophenyl) Quinoline, 4-(4-aminophenyl) Quinoline. Anthracene, Anthraquinone and Phenanthrene, Mater Lett 178:221. https://doi.org/10.1016/j.matlet.2016.04.097
Guezguez I, Ayadi A, Ordon K, Iliopoulos K, Branzea DG, Migalska-Zalas A, Makowska-Janusik M, El-Ghayoury A, Sahraoui B (2014) Zinc induced a dramatic enhancement of the nonlinear optical properties of an Azo-based iminopyridine ligand. J Phys Chem C 118(14):7545. https://doi.org/10.1021/jp412204f
Jensen L, Van Duijnen PT, Snijders JG, Chong DP (2002) Time-dependent density functional study of the static second hyperpolarizability of BB-, NN- and BN-substituted C60. Chem Phys Lett 359(5–6):524. https://doi.org/10.1016/S0009-2614(02)00739-X
Naghavi SS, Gruhn T, Alijani V, Fecher GH, Felser C, Medjanik K, Kutnyakhov D, Nepijko SA, Schönhense G, Rieger R, Baumgarten M, Müllen K (2011) Theoretical study of new acceptor and donor molecules based on polycyclic aromatic hydrocarbons. J Mole Spectrosc 265(2):95. https://doi.org/10.1016/j.jms.2010.12.004
Takeuchi Rudiono M (1997) Improvement of photovoltaic performance in copper phthalocyanine binder layer solar cells. Japan J Appl Phys, Part 2: Lett 36(2A):L127. https://doi.org/10.1143/jjap.36.l127
Tyutyulkov N, Karabunarliev S, Müllen K, Baumgarten M (1993) Band structure of quasi-1D polycondensed hydrocarbons II. Energy spectra of ribbon polym relation to graphite, Synth Metals 53(2):205. https://doi.org/10.1016/0379-6779(93)90891-Y
Friedrich J, Haarer D (1984) Photochemical hole burning: a spectroscopic study of relaxation processes in polymers and glasses. Angewandte Chemie Int Edition in English 23(2):113. https://doi.org/10.1002/anie.198401131
Ekern SP, Marshall AG, Szczepanski J, Vala M (1998) Photodissociation of gas-phase polycylic aromatic hydrocarbon cations. J Phys Chem A 102(20):3498. https://doi.org/10.1021/jp980488e
Choi SH (2017) Unique properties of graphene quantum dots and their applications in photonic/electronic devices. J Phys D: Appl Phys 50(10):103002. https://doi.org/10.1088/1361-6463/aa5244
Richter M, Heumüller T, Matt GJ, Heiss W, Brabec CJ (2017) Carbon Photodetectors: the versatility of carbon allotropes. Adv Energy Mater 7(10):1601574. https://doi.org/10.1002/aenm.201601574
Sancho-García JC, Pérez-Jiménez AJ (2014) Theoretical study of stability and charge-transport properties of coronene molecule and some of its halogenated derivatives: a path to ambipolar organic-based materials? J Chem Phys 141(13):134708. https://doi.org/10.1063/1.4897205
Wang L, Li P, Xu B, Zhang H, Tian W (2014) The substituent effect on charge transport property of triisopropylsilylethynyl anthracene derivatives. Organ Electron 15(10):2476. https://doi.org/10.1016/j.orgel.2014.07.003
de Proft F, Geerlings P (2001) Conceptual and computational DFT in the study of aromaticity. Chem. Rev. 101(5):1451
Zhan CG, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107(20):4184. https://doi.org/10.1021/jp0225774
Arulmozhiraja S, Fujii T, Tokiwa H (2000) Electron affinity for the most toxic 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): a density functional theory study. J Phys Chem A 104(30):7068. https://doi.org/10.1021/jp994237x
Hush NS (1958) Adiabatic rate processes at electrodes. I. Energy-charge relationships. J Chem Phys 28(5):962. https://doi.org/10.1063/1.1744305
Marcus RA (1993) Electron transfer reactions in chemestry. Theory and exp, Rev Modern Phys 65:599
Gao H, Qin C, Zhang H, Wu S, Su ZM, Wang Y (2008) Theoretical characterization of a typical hole/exciton-blocking material bathocuproine and its analogues. J Phys Chem A 112(38):9097. https://doi.org/10.1021/jp804308e
Feller D (1996) Computational chemistry calculations. J Comput Chem 17(13):1571
Schuchardt KL, Didier BT, Elsethagen T, Sun L, Gurumoorthi V, Chase J, Li J, Windus TL (2007) Basis set exchange: a community database for computational sciences. J Chem Inform Model 47(3):1045. https://doi.org/10.1021/ci600510j
Chai MHGJ (2008) Long-range corrected hybrid density functionals with improved dispersion corrections. J Chemi Theory Comput 9(1):263. https://doi.org/10.1021/ct300715s
Holtomo O, Nsangou M, Fifen JJ, Motapon O (2019) Thermodynamic of solvation, solute - Solvent electron transfer and ionization potential of BSCAPE molecule and its UV-vis spectra in aqueous solution. J Mole Graph Model 92:100. https://doi.org/10.1016/j.jmgm.2019.06.016
Chen XK, Zou LY, Huang S, Min CG, Ren AM, Feng JK, Sun CC (2011) Theoretical investigation of charge injection and transport properties of novel organic semiconductor materials - Cyclic oligothiophenes. Organ Electron 12(7):1198. https://doi.org/10.1016/j.orgel.2011.03.029
Datta A, Mohakud S, Pati SK (2007) Comparing the electron and hole mobilities in the \(\alpha \) and \(\beta \) phases of perylene: Role of \(\pi \)-stacking. J Mater Chem 17(19):1933. https://doi.org/10.1039/b700625j
Mohakud S, Alex AP, Pati SK (2010) Ambipolar charge transport in \(\alpha \)-oligofurans: a theoretical study. J Phys Chem C 114(48):20436. https://doi.org/10.1021/jp1047503
Guo Y, Wang W, Shao R, Yin S (2015) Theoretical study on the electron transport properties of chlorinated pentacene derivatives. Comput Theoretical Chem 1057:67. https://doi.org/10.1016/j.comptc.2015.01.019
Carmen Ruiz Delgado M, Kim EG, Da Silva Filho DA, Bredas JL (2010) Tuning the charge-transport parameters of perylene diimide single crystals via end and/or core functionalization: a density functional theory investigation. J Am Chem Soc 132(10):3375. https://doi.org/10.1021/ja908173x
Song P, Fengcai M (2010) Tunable electronic structures and optical properties of fluorenone-based molecular materials by heteroatoms. J Phys Chem A 114(5):2230. https://doi.org/10.1021/jp909594e
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Petersson G, Nakatsuji H et al (2016) Gaussian 16
Brown Js, (2018). catnip (version 1.9). [software]. available from https://github.com/joshuasbrown/qc_tools
Valeev EF, Coropceanu V, Da Silva Filho DA, Salman S, Brédas JL (2006) Effect of electronic polarization on charge-transport parameters in molecular organic semiconductors. J Am Chem Soc 128(30):9882. https://doi.org/10.1021/ja061827h
Baumeier B, Kirkpatrick J, Andrienko D (2010) Density-functional based determination of intermolecular charge transfer properties for large-scale morphologies. Phys Chem Chem Phys 12(36):11103. https://doi.org/10.1039/c002337j
Baird T, Gall JH, MacNicol DD, Mallinson PR, Michie CR (1988) Perchlorocoronene: a novel host precursor. J Chem Soc, Chem Commun 22:1471. https://doi.org/10.1039/C39880001471
Erkoç S, Erkoç F, Türker L (2000) Structural and electronic properties of halogenated coronene. J Mole Struct 538(1–3):91. https://doi.org/10.1016/S0166-1280(00)00651-5
Zhao C, Guo Y, Guan L, Ge H, Yin S, Wang W (2014) Theoretical investigation on charge transport parameters of two novel heterotetracenes as ambipolar organic semiconductors. Synthetic Metals 188:146. https://doi.org/10.1016/j.synthmet.2013.12.009
Yin J, Chaitanya K, Ju XH (2015) Structures and charge transport properties of selenosulflower and its selenium analogue selflower: computer-aided design of high-performance ambipolar organic semiconductors. J Mater Chem C 3(14):3472. https://doi.org/10.1039/c4tc02655a
Chen L, Xu C, Zhang XF (2008) DFT calculations of vibrational spectra and nonlinear optical properties for MgO nanotube clusters. J Mole Struct: THEOCHEM 863(1–3):55. https://doi.org/10.1016/j.theochem.2008.05.020
Jamróz MH (2013) Vibrational energy distribution analysis (VEDA): scopes and limitations. Spectrochimica Acta - Part A: Mole Biomol Spectrosc 114:220. https://doi.org/10.1016/j.saa.2013.05.096
Zhang SF, Chen XK, Fan JX, Ren AM (2013) Charge transport properties in a series of five-ring-fused thienoacenes: a quantum chemistry and molecular mechanic study. Organ Electron 14(2):607. https://doi.org/10.1016/j.orgel.2012.12.001
Heyd J, Scuseria GE, Ernzerhof M (2003) Hybrid functionals based on a screened Coulomb potential. J Chem Phys 118(18):8207. https://doi.org/10.1063/1.1564060
Wang L, Huang W, Li R, Gehrig D, Blom PW, Landfester K, Zhang KA (2016) Structural design principle of small-molecule organic semiconductors for metal-free. Visible-Light-Promoted Photocatal, Angewandte Chemie - Int Edition 55(33):9783. https://doi.org/10.1002/anie.201603789
Saranya G, Navamani K, Senthilkumar K (2014) A theoretical study on optical and charge transport properties of anthra-[1,2-b:4,3-b...:5,6-b...:8,7-bâ] tetrathiophene molecules. Chem Phys 433:48. https://doi.org/10.1016/j.chemphys.2014.01.020
Adiga SP, Shukla D (2010) Electronic structure and charge-transport properties of N, N-Bis(cyclohexyl)naphthalene Diimide. J Phys Chem 114:2751
Schroeder PG, France CB, Parkinson BA, Schlaf R (2002) Orbital alignment at p-sexiphenyl and coronene/layered materials interfaces measured with photoemission spectroscopy. J Appl Phys 91(11):9095. https://doi.org/10.1063/1.1473217
Duncan MA, Knight AM, Negishi Y, Nagao S, Nakamura Y, Kato A, Nakajima A, Kaya K (1999) Production of jet-cooled coronene and coronene cluster anions and their study with photoelectron spectroscopy. Chem Phys Lett 309(1–2):49. https://doi.org/10.1016/S0009-2614(99)00662-4
Shi Y, Shi Y, Wei H, Zhai H, Liu Y (2017) A theoretical study on the electronic properties of two ring-fused derivatives of 9,10-diphenylanthracene. New J Chem 41(18):10251. https://doi.org/10.1039/c7nj02590d
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580. https://doi.org/10.1002/jcc.22885
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104. https://doi.org/10.1063/1.3382344
Hudgins DM, Sandford SA (2000) Infrared spectroscopy of matrix isolated polycyclic aromatic hydrocarbons. 2. PAHs Containing Five or. More Rings 5639(98):344
Patterson JW (1942) The ultraviolet absorption spectra of coronene. J Am Chem Soc 64(6):1485. https://doi.org/10.1021/ja01258a505
Acknowledgements
The authors are grateful to the Center for High Performance Computing (CHPC), South Africa, for granting them access to their clusters and computational resources.
Funding
No funds or grants were received.
Author information
Authors and Affiliations
Contributions
Marius Bouba Ousmanou: conceptualization; investigation; methodology; formal analysis; writing-original draft. Fridolin Tchangnwa Nya: conceptualization; investigation; methodology; writing-review and editing; supervision. Alhadji Malloum: writing-review and editing. Jeanet Conradie: writing-review and editing. Jean Marie Ndjaka: writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies involving animals performed by any of the author.
Consent to participate
All the co-authors consent to participate.
Consent to publish
All the co-authors consent to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bouba, M.O., Tchangnwa Nya, F., Malloum, A. et al. DFT investigation of Percyanation effect of coronene molecule: Comparative study with their Perhalogenated counterparts.. Polym. Bull. 79, 9663–9684 (2022). https://doi.org/10.1007/s00289-021-03967-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03967-5