Abstract
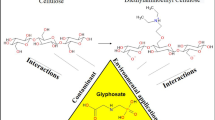
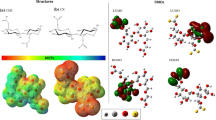
This study provides theoretical insights into the potential use of cellulose derivatives, such as methylcellulose (ME) and cellulose xanthate (CX), to remove glyphosate (GLY) contaminants via adsorption. The mechanism of adsorption in ME and CX is compared with that of activated carbon. To this end, theoretical calculations based on density functional theory (DFT) were used to determine the frontier molecular orbitals (FMOs); molecular electrostatic potential (MEP); and energetic, structural, and topological parameters. The analyses of FMOs and MEP indicated two possible interaction sites. The structural parameters showed that the herbicide interacts with the ME and CX matrices, and the bond lengths of the interaction ranging from 1.58 to 3.09 Å, depending on the nature of the interaction. The vibrational frequencies of the bonds involved in the interaction changed after adsorption, thus confirming the existence of the interaction. The analysis of the quantum theory of atoms in molecules (QTAIM) allowed the characterization of interactions through topological parameters and showed that the most effective interactions presented a higher number of electrostatic interactions. The determined energies of the electronic interaction and the enthalpy were negative, indicating that the interaction occurred. Finally, the calculations for the glyphosate adsorption process on activated carbon (AC) showed that the terminal group –COOH presented the best energy values for interaction with GLY, followed by activated carbon with the group –OH, and finally, the activated carbon containing only aromatic rings. The results showed that the derivatives of cellulose CX and ME are promising alternatives to remove glyphosate contaminants from water.





Similar content being viewed by others
References
Grossbard E, Atkinson DC (1985) The herbicide glyphosate. BUTTERWORTH
Turner D (1985) Effects on glyphosate performance of formulation, additives and mixing with other herbicides, The Herbicide Glyphosate.
Tsui MTK, Chu LM (2003) Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52:1189–1197. https://doi.org/10.1016/S0045-6535(03)00306-0
Richmond ME (2018) Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J Environ Stud Sci 8:416–434. https://doi.org/10.1007/s13412-018-0517-2
PubChem, Hazardous Substances Data Bank (HSDB) : 3432, (n.d.). https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3432. Accessed 20 Jan 2021
Nandula VK (2010) Glyphosate Resistance in Crops and Weeds: History, Development, and Management. Wiley, New York
de Aguiar Filho SQ, Costa AMF, dos Santos Pereira AK et al (2021) Interaction of glyphosate in matrices of cellulose and diethylaminoethyl cellulose biopolymers: theoretical viewpoint of the adsorption process. J Mol Model 27:272. https://doi.org/10.1007/s00894-021-04894-y
Van Bruggen AHC, He MM, Shin K, Mai V, Jeong KC, Finckh MR, Morris JG (2018) Environmental and health effects of the herbicide glyphosate. Sci Total Environ 616–617:255–268. https://doi.org/10.1016/j.scitotenv.2017.10.309
Mañas F, Peralta L, Raviolo J, Ovando HG, Weyers A, Ugnia L, Cid MG, Larripa I, Gorla N (2009) Genotoxicity of glyphosate assessed by the comet assay and cytogenetic tests. Environ Toxicol Pharmacol 28:37–41. https://doi.org/10.1016/j.etap.2009.02.001
Thongprakaisang S, Thiantanawat A, Rangkadilok N, Suriyo T, Satayavivad J (2013) Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem Toxicol 59:129–136. https://doi.org/10.1016/j.fct.2013.05.057
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Some organophosphate insecticides and herbicides, 2017. http://www.ncbi.nlm.nih.gov/books/NBK436774/. Accessed October 10, 2020.
Kwiatkowska M, Reszka E, Woźniak K, Jabłońska E, Michałowicz J, Bukowska B (2017) DNA damage and methylation induced by glyphosate in human peripheral blood mononuclear cells (in vitro study). Food Chem Toxicol 105:93–98. https://doi.org/10.1016/j.fct.2017.03.051
Duforestel M, Nadaradjane A, Bougras-Cartron G, Briand J, Olivier C, Frenel J-S, Vallette FM, Lelièvre SA, Cartron P-F (2019) Glyphosate primes mammary cells for tumorigenesis by reprogramming the epigenome in a TET3-dependent manner. Front Genet 10. https://doi.org/10.3389/fgene.2019.00885.
Castiglioni S, Bagnati R, Fanelli R, Pomati F, Calamari D, Zuccato E (2006) Removal of pharmaceuticals in sewage treatment plants in Italy. Environ Sci Technol 40:357–363. https://doi.org/10.1021/es050991m
Choy KKH, McKay G, Porter JF (1999) Sorption of acid dyes from effluents using activated carbon. Resources Conserv Recycling 27:57–71. https://doi.org/10.1016/S0921-3449(98)00085-8
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Madhav S, Ahamad A, Singh P, Mishra PK (2018) A review of textile industry: wet processing, environmental impacts, and effluent treatment methods. Environ Qual Manage 27:31–41. https://doi.org/10.1002/tqem.21538
Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, vom Saal FS, Welshons WV, Benbrook CM (2016) Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health 15:19. https://doi.org/10.1186/s12940-016-0117-0
Feng D, Malleret L, Soric A, Boutin O (2020) Kinetic study of glyphosate degradation in wet air oxidation conditions. Chemosphere 247:125930. https://doi.org/10.1016/j.chemosphere.2020.125930
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24. https://doi.org/10.1016/j.cej.2009.10.029
Resende RF, Leal PVB, Pereira DH, Papini RM, Magriotis ZM (2020) Removal of fatty acid by natural and modified bentonites: elucidation of adsorption mechanism. Colloids Surfaces A Physicochem Eng Aspects 605:125340. https://doi.org/10.1016/j.colsurfa.2020.125340
Hameed BH, Krishni RR, Sata SA (2009) A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J Hazard Mater 162:305–311. https://doi.org/10.1016/j.jhazmat.2008.05.036
Dai Y, Sun Q, Wang W, Lu L, Liu M, Li J, Yang S, Sun Y, Zhang K, Xu J, Zheng W, Hu Z, Yang Y, Gao Y, Chen Y, Zhang X, Gao F, Zhang Y (2018) Utilizations of agricultural waste as adsorbent for the removal of contaminants: a review. Chemosphere 211:235–253. https://doi.org/10.1016/j.chemosphere.2018.06.179
Geethakarthi A, Phanikumar BR (2010) Industrial sludge based adsorbents/ industrial by-products in the removal of reactive dyes A review. IJWREE 3:1–9. https://doi.org/10.5897/IJWREE.9000029
Ribeiro IHS, Reis DT, Pereira DH (2019) A DFT-based analysis of adsorption of Cd2+, Cr3+, Cu2+, Hg2+, Pb2+, and Zn2+, on vanillin monomer: a study of the removal of metal ions from effluents. J Mol Model 25:267. https://doi.org/10.1007/s00894-019-4151-z
Reis DT, Ribeiro IHS, Pereira DH (2020) DFT study of the application of polymers cellulose and cellulose acetate for adsorption of metal ions (Cd2+, Cu2+ and Cr3+) potentially toxic. Polym Bull 77:3443–3456. https://doi.org/10.1007/s00289-019-02926-5
Menazea AA, Ezzat HA, Omara W, Basyouni OH, Ibrahim SA, Mohamed AA, Tawfik W, Ibrahim MA (2020) Chitosan/graphene oxide composite as an effective removal of Ni, Cu, As, Cd and Pb from wastewater. Comput Theor Chem 1189:112980. https://doi.org/10.1016/j.comptc.2020.112980
Zhou S, Sun X, Jiang G (2021) A DFT study on the adsorption of nucleobases with Au20. J Mol Model 27:29. https://doi.org/10.1007/s00894-020-04618-8
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871. https://doi.org/10.1103/PhysRev.136.B864
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. https://doi.org/10.1103/PhysRev.140.A1133
Parr RG, Yang W (1989) Density functional theory of atoms and molecules, vol 1. OXford University Press, Oxford, p 989.
Becke AD (2014) Perspective: fifty years of density-functional theory in chemical physics. J Chem Phys 140:18A301. https://doi.org/10.1063/1.4869598
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620. https://doi.org/10.1039/B810189B
Ditchfield R, Hehre WJ, Pople JA (1971) Self‐consistent molecular‐orbital methods. IX. An extended Gaussian‐type basis for molecular‐orbital studies of organic molecules. J Chem Phys 54:724–728. https://doi.org/10.1063/1.1674902.
Hehre WJ, Ditchfield R, Pople JA (1972) Self—Consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527.
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theoret Chim Acta 28:213–222. https://doi.org/10.1007/BF00533485
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal Solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Dennington R, Keith T, Millam JG (2009) Gauss View, Version 5; Semichem Inc: Shawnee Mission
Frisch J, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian09, Revision D.01, Gaussian, Inc., Wallingford.
Bader RFW, Essén H (1984) The characterization of atomic interactions. J Chem Phys 80:1943–1960. https://doi.org/10.1063/1.446956
Bader RFW (1990) Atoms in molecules: a quantum theory, 1st edn. Oxford University Press, Oxford
Keith TA, Bader RFW, Aray Y (1996) Structural homeomorphism between the electron density and the virial field. Int J Quantum Chem 57:183–198. https://doi.org/10.1002/(SICI)1097-461X(1996)57:2%3c183::AID-QUA4%3e3.0.CO;2-U
Popelier PLA (1999) Quantum Molecular Similarity. 1. BCP Space. J Phys Chem A 103:2883–2890. https://doi.org/10.1021/jp984735q.
Todd A, Keith T (2017), AIMAll (Version 10.05. 04), Gristmill Software, Overland Park KS, USA
Mohamed Naseer Ali M, Kaliannan P, Venuvanalingam P (2005) Conformation and function of N-hydroxy-glyphosate and N-amino-glyphosate: a comparative study using ab initio MO theory. J Molecular Structure THEOCHEM 714(2005):99–108. https://doi.org/10.1016/j.theochem.2004.10.026
Kaliannan P, Mohamed Naseer Ali M, Seethalakshmi T, Venuvanalingam P (2002) Electronic structure and conformation of glyphosate: an ab initio MO study. J Molecular Structure THEOCHEM. 618:117–125. https://doi.org/10.1016/S0166-1280(02)00467-0.
Costa MPM, Prates LM, Baptista L, Cruz MTM, Ferreira ILM (2018) Interaction of polyelectrolyte complex between sodium alginate and chitosan dimers with a single glyphosate molecule: A DFT and NBO study. Carbohyd Polym 198:51–60. https://doi.org/10.1016/j.carbpol.2018.06.052
Ahmed AA, Gros P, Kühn O, Leinweber P (2018) Molecular level investigation of the role of peptide interactions in the glyphosate analytics. Chemosphere 196:129–134. https://doi.org/10.1016/j.chemosphere.2017.12.162
Costa AMF, de Aguiar Filho SQ, Santos TJ, Pereira DH (2021) Theoretical insights about the possibility of removing Pb2+ and Hg2+ metal ions using adsorptive processes and matrices of carboxymethyl diethylaminoethyl cellulose and cellulose nitrate biopolymers. J Molecular Liquids. 331:115730. https://doi.org/10.1016/j.molliq.2021.115730.
Sprankle P, Meggitt WF, Penner D (1975) Adsorption, mobility, and microbial degradation of glyphosate in the soil. Weed Sci 23:229–234
Reis DT, Pereira AKS, Scheidt GN, Pereira DH (2019) Plant and bacterial cellulose: production, chemical structure, derivatives and applications, orbital. Electronic J Chem 11:321–329. https://doi.org/10.17807/orbital.v11i5.1349
Reis DT, de Aguiar Filho SQ, Grotto CGL, Bihain MFR, Pereira DH (2020) Carboxymethylcellulose and cellulose xanthate matrices as potential adsorbent material for potentially toxic Cr3+, Cu2+ and Cd2+metal ions: a theoretical study. Theor Chem Acc 139:96. https://doi.org/10.1007/s00214-020-02610-2.
Melchor-Rodríguez K, Gaspard S, Jáuregui-Haza U (2020) Chlordecone adsorption on functionalized activated carbons: computational chemistry as tool for understanding the adsorption process. Quím. Nova. https://doi.org/10.21577/0100-4042.20170666.
Carneiro RTA, Taketa TB, Gomes Neto RJ, Oliveira JL, Campos EVR, de Moraes MA, da Silva CMG, Beppu MM, Fraceto LF (2015) Removal of glyphosate herbicide from water using biopolymer membranes. J Environ Manae 151:353–360. https://doi.org/10.1016/j.jenvman.2015.01.005.
Moradeeya PG, Kumar MA, Thorat RB, Rathod M, Khambhaty Y, Basha S (2017) Nanocellulose for biosorption of chlorpyrifos from water: chemometric optimization, kinetics and equilibrium. Cellulose 24:1319–1332. https://doi.org/10.1007/s10570-017-1197-x
Rissouli l, Benicha M, Chafik T, Chabbi D (2017) Decontamination of water polluted with pesticide using biopolymers: adsorption of glyphosate by chitin and chitosan. JMES 8:4544–4549. https://doi.org/10.26872/jmes.2017.8.12.479.
Desbrières J, Guibal E (2018) Chitosan for wastewater treatment: Chitosan for wastewater treatment. Polym Int 67:7–14. https://doi.org/10.1002/pi.5464
Sanchez LM, Ollier RP, Pereira AES, Fraceto L, Alvarez VA (2020) Pesticide removal from industrial effluents using biopolymeric materials. In: Biopolymer Membranes and Films. Elsevier, Amsterdam, pp 359–382
Acknowledgements
The authors acknowledge the Center for Computational Engineering and Sciences (Financial support from FAPESP Fundação de Amparo à Pesquisa, Grant 2013/08293-7, and Grant 2017/11485-6) and the National Center for High Performance Processing (Centro Nacional de Processamento de Alto Desempenho—CENAPAD) in São Paulo, UNICAMP (Universidade Estadual de Campinas), for computational resources. The authors would also like to acknowledge funding from CAPES (Coordination of Improvement of Higher Education Personnel—Brazil, Funding Code 001 CAPES) and the PROPESQ/Federal University of Tocantins (Edital Nº29 /2020 para tradução de artigos científicios da Universidade Federal do Tocantins—PROPESQ/UFT). AKSP thank to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), scholarships # 164658/2018-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Aguiar Filho, S.Q., dos Santos Pereira, A.K., Cavallini, G.S. et al. Theoretical study of glyphosate adsorption potential on methylcellulose and cellulose xanthate matrices compared to activated carbon: role of biopolymers in the adsorption process. Polym. Bull. 79, 9331–9344 (2022). https://doi.org/10.1007/s00289-021-03957-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03957-7