Abstract
Glyphosate is an herbicide widely used in agricultural activities causing contamination of soils and bodies of water and damage to the biodiversity of ecosystems. In this context, the present study aimed to theoretically study the adsorption potential of the biopolymer cellulose (CE) and its diethylaminoethyl cellulose derivative (DEAEC) with the herbicide glyphosate (GLY). Theoretical calculations were performed using the density functional theory. Molecular electrostatic potential and frontier molecular orbital analyses were performed, which allowed identifying the possible sites of interaction of biopolymers that were in the functional groups –OH and O− of cellulose and in the groups –O− and –NH+(CH2CH3)2 of the DEAEC. Reactivity indices chemical softness and hardness showed that both adsorbents could interact with adsorbate. Simulated IR indicated that the interactions could be evinced in experimental measurements by changes in the bands of glyphosate (ν(P = O), δ(P-O–H), δ(C-N–H)) or in the bands of CE and DEAEC (ν(C–O), ν(C–H), ν(N–H)). The binding energies showed that the GLY interacts more effectively with CE than DEAEC. The ΔH prove that all processes are exothermic and the CE-GLY1 interaction showed value of ΔG < 0. The topological results showed a greater number of interactions with electrostatic nature. The results found in the study show that the theoretical data provides useful information to support the use of biopolymers as matrices for glyphosate adsorption or other contaminants.
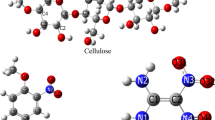
Graphical abstract






Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Nakka S, Jugulam M, Peterson D, Asif M (2019) Herbicide resistance: development of wheat production systems and current status of resistant weeds in wheat cropping systems. The Crop Journal, Breeding wheat for the global north: China, the USA and Canada 7:750–760. https://doi.org/10.1016/j.cj.2019.09.004
Oerke E-C (2006) Crop losses to pests. J Agric Sci 144:31–43. https://doi.org/10.1017/S0021859605005708
Croll BT (1991) Pesticides in surface waters and groundwaters. Water and Environment Journal 5:389–395. https://doi.org/10.1111/j.1747-6593.1991.tb00635.x
Ollinger M, Aspelin A, Shields M (1998) US regulation and new pesticide registrations and sales. Agribusiness 14:199–212. https://doi.org/10.1002/(SICI)1520-6297(199805/06)14:3%3c199::AID-AGR3%3e3.0.CO;2-W
Pelaez V, da Silva LR, Araújo EB (2013) Regulation of pesticides: a comparative analysis. Sci Public Policy 40:644–656. https://doi.org/10.1093/scipol/sct020
Ramrakhiani L, Ghosh S, Mandal A, Majumdar S (2019) Utilization of multi-metal laden spent biosorbent for removal of glyphosate herbicide from aqueous solution and its mechanism elucidation. Chem Eng J 361:1063–1077. https://doi.org/10.1016/j.cej.2018.12.163
Fiorilli S, Rivoira L, Calì G, Appendini M, Bruzzoniti MC, Coïsson M, Onida B (2017) Iron oxide inside SBA-15 modified with amino groups as reusable adsorbent for highly efficient removal of glyphosate from water. Appl Surf Sci 411:457–465. https://doi.org/10.1016/j.apsusc.2017.03.206
Feng D, Malleret L, Soric A, Boutin O (2020) Kinetic study of glyphosate degradation in wet air oxidation conditions. Chemosphere 247:125930. https://doi.org/10.1016/j.chemosphere.2020.125930
Gros P, Ahmed A, Kühn O, Leinweber P (2017) Glyphosate binding in soil as revealed by sorption experiments and quantum-chemical modeling. Sci Total Environ 586:527–535. https://doi.org/10.1016/j.scitotenv.2017.02.007
World Health Organization (WHO) (2016). World https://www.who.int/foodsafety/jmprsummary2016.pdf?ua=1. Accessed 19 Jul 2021
Castiglioni S, Bagnati R, Fanelli R, Pomati F, Calamari D, Zuccato E (2006) Removal of pharmaceuticals in sewage treatment plants in Italy. Environ Sci Technol 40:357–363. https://doi.org/10.1021/es050991m
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Crini G, Lichtfouse E, Wilson LD, Morin-Crini N (2019) Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett 17:195–213. https://doi.org/10.1007/s10311-018-0786-8
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243. https://doi.org/10.1016/S0304-3894(02)00263-7
Ince M, Kaplan Ince O (2017) An overview of adsorption technique for heavy metal removal from water/wastewater: a critical review. International Journal of Pure and Applied Sciences 3:10–19. https://doi.org/10.29132/ijpas.358199
Abu-Dalo MA, Nevostrueva S, Hernandez M (2020) Removal of radionuclides from acidic solution by activated carbon impregnated with methyl- and carboxy-benzotriazoles. Sci Rep 10:11712. https://doi.org/10.1038/s41598-020-68645-4
Hameed BH, Krishni RR, Sata SA (2009) A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J Hazard Mater 162:305–311. https://doi.org/10.1016/j.jhazmat.2008.05.036
Dai Y, Sun Q, Wang W, Lu L, Liu M, Li J, Yang S, Sun Y, Zhang K, Xu J, Zheng W, Hu Z, Yang Y, Gao Y, Chen Y, Zhang X, Gao F, Zhang Y (2018) Utilizations of agricultural waste as adsorbent for the removal of contaminants: a review. Chemosphere 211:235–253. https://doi.org/10.1016/j.chemosphere.2018.06.179
Geethakarthi A, Phanikumar BR (2010) Industrial sludge based adsorbents/industrial by-products in the removal of reactive dyes A review. IJWREE 3:1–9. https://doi.org/10.5897/IJWREE.9000029
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24. https://doi.org/10.1016/j.cej.2009.10.029
Resende RF, Leal PVB, Pereira DH, Papini RM, Magriotis ZM (2020) Removal of fatty acid by natural and modified bentonites: elucidation of adsorption mechanism. Colloids Surf, A 605:125340. https://doi.org/10.1016/j.colsurfa.2020.125340
Reis DT, Ribeiro IHS, Pereira DH (2020) DFT study of the application of polymers cellulose and cellulose acetate for adsorption of metal ions (Cd2+, Cu2+ and Cr3+) potentially toxic. Polym Bull 77:3443–3456. https://doi.org/10.1007/s00289-019-02926-5
Ribeiro IHS, Reis DT, Pereira DH (2019) A DFT-based analysis of adsorption of Cd2+, Cr3+, Cu2+, Hg2+, Pb2+, and Zn2+, on vanillin monomer: a study of the removal of metal ions from effluents. J Mol Model 25:267. https://doi.org/10.1007/s00894-019-4151-z
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresource Technology 99:6709–6724. https://doi.org/10.1016/j.biortech.2008.01.036
Suhas Gupta VK, Carrott PJM, Singh R, Chaudhary M, Kushwaha S (2016) Cellulose: a review as natural, modified and activated carbon adsorbent. Biores Technol 216:1066–1076. https://doi.org/10.1016/j.biortech.2016.05.106
Rahman NSA, Yhaya MF, Azahari B, Ismail WR (2018) Utilisation of natural cellulose fibres in wastewater treatment. Cellulose 25:4887–4903. https://doi.org/10.1007/s10570-018-1935-8
Varghese AG, Paul SA, Latha MS (2019) Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ Chem Lett 17:867–877. https://doi.org/10.1007/s10311-018-00843-z
Fischer S, Thümmler K, Volkert B, Hettrich K, Schmidt I, Fischer K (2008) Properties and applications of cellulose acetate. Macromol Symp 262:89–96. https://doi.org/10.1002/masy.200850210
Yang XH, Zhu WL (2007) Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 14:409–417. https://doi.org/10.1007/s10570-007-9137-9
Morozova S (2020) Methylcellulose fibrils: a mini review. Polym Int 69:125–130. https://doi.org/10.1002/pi.5945
Pourmortazavi SM, Hosseini SG, Rahimi-Nasrabadi M, Hajimirsadeghi SS, Momenian H (2009) Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater 162:1141–1144. https://doi.org/10.1016/j.jhazmat.2008.05.161
Menefee E, Hautala E (1978) Soil stabilisation by cellulose xanthate. Nature 275:530–532. https://doi.org/10.1038/275530a0
Beyki MH, Bayat M, Miri S, Shemirani F, Alijani H (2014) Synthesis, characterization, and silver adsorption property of magnetic cellulose xanthate from acidic solution: prepared by one step and biogenic approach. Ind Eng Chem Res 53:14904–14912. https://doi.org/10.1021/ie501989q
Frank RA, Kavanagh R, Burnison BK, Headley JV, Peru KM, Der Kraak GV, Solomon KR (2006) Diethylaminoethyl-cellulose clean-up of a large volume naphthenic acid extract. Chemosphere 64:1346–1352. https://doi.org/10.1016/j.chemosphere.2005.12.035
Heri W, Neukom H, Deuel H (1961) Chromatographische Fraktionierung von Pektinstoffen an Diäthylaminoäthyl-Cellulose. 15. Mitteilung über Ionenaustauscher Helvetica Chimica Acta 44:1939–1945. https://doi.org/10.1002/hlca.19610440715
Smit CJB, Bryant EF (1967) Properties of pectin fractions separated on diethylaminoethyl-cellulose Columns. J Food Science 32:197–199. https://doi.org/10.1111/j.1365-2621.1967.tb01292.x
Reis DT, de Aguiar Filho SQ, Grotto CGL, Bihain MFR, Pereira DH (2020) Carboxymethylcellulose and cellulose xanthate matrices as potential adsorbent material for potentially toxic Cr3+, Cu2+ and Cd2+metal ions: a theoretical study. Theor Chem Acc 139:96. https://doi.org/10.1007/s00214-020-02610-2
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871. https://doi.org/10.1103/PhysRev.136.B864
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. https://doi.org/10.1103/PhysRev.140.A1133
Parr RG (1989) W. Yang Density functional theory of atoms and molecules. Oxford University Press 1, 1989
Becke AD (2014) Perspective: fifty years of density-functional theory in chemical physics. J Chem Phys 140:18A301. https://doi.org/10.1063/1.4869598
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620. https://doi.org/10.1039/B810189B
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728. https://doi.org/10.1063/1.1674902
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theoret Chim Acta 28:213–222. https://doi.org/10.1007/BF00533485
Liu Y, Liu Y, Gallo AA, Knierim KD, Taylor ER, Tzeng N (2015) Performances of DFT methods implemented in G09 for simulations of the dispersion-dominated CH-π in ligand–protein complex: a case study with glycerol-GDH. J Mol Struct 1084:223–228. https://doi.org/10.1016/j.molstruc.2014.12.028
Matczak P (2015) Assessment of various density functionals for intermolecular N→Sn interactions: the test case of poly(trimethyltin cyanide). Comput Theor Chem 1051:110–122. https://doi.org/10.1016/j.comptc.2014.10.028
Rayne S, Forest K (2016) A comparative examination of density functional performance against the ISOL24/11 isomerization energy benchmark. Comput Theor Chem 1090:147–152. https://doi.org/10.1016/j.comptc.2016.06.018
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Costa AMF, de Aguiar Filho SQ, Santos TJ, Pereira DH (2021) Theoretical insights about the possibility of removing Pb2+ and Hg2+ metal ions using adsorptive processes and matrices of carboxymethyldiethylaminoethyl cellulose and cellulose nitrate biopolymers. J Mol Liq 331:115730. https://doi.org/10.1016/j.molliq.2021.115730
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807. https://doi.org/10.1063/1.436185
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE Jr, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D01, Gaussian, Inc., Wallingford, CT, 2009
Dennington R, Keith T, Millam JG (2009) Gauss View, Version 5. Semichem Inc., Shawnee Mission
Bader RFW, Essén H (1984) The characterization of atomic interactions. J Chem Phys 80:1943–1960. https://doi.org/10.1063/1.446956
Bader RFW (1990) Atoms in molecules: a quantum theory, 1st edn. Oxford Univ. Press, Oxford
Keith TA, Bader RFW, Aray Y (1996) Structural homeomorphism between the electron density and the virial field. Int J Quantum Chem 57:183–198. https://doi.org/10.1002/(SICI)1097-461X(1996)57:2%3c183::AID-QUA4%3e3.0.CO;2-U
Popelier PLA (1999) Quantum molecular similarity. 1. BCP Space J Phys Chem A 103:2883–2890. https://doi.org/10.1021/jp984735q
Kumar PSV, Raghavendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 128:1527–1536. https://doi.org/10.1007/s12039-016-1172-3
Todd A, Keith T (2017) AIMAll (Version 10.05. 04). Gristmill Software, Overland Park KS, USA.
Yakout AA, El-Sokkary RH, Shreadah MA, Abdel Hamid OG (2016) Removal of Cd(II) and Pb(II) from wastewater by using triethylenetetramine functionalized grafted cellulose acetate-manganese dioxide composite. Carbohyd Polym 148:406–414. https://doi.org/10.1016/j.carbpol.2016.04.038
Kenawy IM, Hafez MAH, Ismail MA, Hashem MA (2018) Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from aqueous single metal solutions by guanyl-modified cellulose. Int J Biol Macromol 107:1538–1549. https://doi.org/10.1016/j.ijbiomac.2017.10.017
Khademian E, Salehi E, Sanaeepur H, Galiano F, Figoli A (2020) A systematic review on carbohydrate biopolymers for adsorptive remediation of copper ions from aqueous environments-part A: classification and modification strategies. Sci Total Environ 738:139829. https://doi.org/10.1016/j.scitotenv.2020.139829
Pearson RG (1968) Hard and soft acids and bases, HSAB, part 1: fundamental principles. J Chem Educ 45:581. https://doi.org/10.1021/ed045p581
Pearson RG (1968) Hard and soft acids and bases, HSAB, part II: underlying theories. J Chem Educ 45:643. https://doi.org/10.1021/ed045p643
Leal PVB, Pereira DH, Papini RM, Magriotis ZM (2021) Effect of dimethyl sulfoxide intercalation into kaolinite on etheramine adsorption: experimental and theoretical investigation. J Environ Chem Eng 9:105503. https://doi.org/10.1016/j.jece.2021.105503
Undabeytia T, Morillo E, Maqueda C (2002) FTIR study of glyphosate−copper complexes. J Agric Food Chem 50:1918–1921. https://doi.org/10.1021/jf010988w
Piccolo A, Celano G (1993) Modification of infrared spectra of the herbicide glyphosate induced by pH variation. J of Env Sc & Hlth, Part B 28:447–457. https://doi.org/10.1080/03601239309372835
de Santana H, Toni LRM, de BenetoliB LO et al (2006) Effect in glyphosate adsorption on clays and soils heated and characterization by FT–IR spectroscopy. Geoderma 136:738–750. https://doi.org/10.1016/j.geoderma.2006.05.012
Subramaniam V, Hoggard PE (1988) Metal complexes of glyphosate. J Agric Food Chem 36:1326–1329. https://doi.org/10.1021/jf00084a050
Miano TM, Piccolo A, Celano G, Senesi N (1992) Infrared and fluorescence spectroscopy of glyphosate-humic acid complexes. Sci Total Environ 123–124:83–92. https://doi.org/10.1016/0048-9697(92)90135-F
Soliman SM, Barakat A, Islam MS, Ghabbour HA (2018) Synthesis, crystal structure and DFT studies of a new dinuclear Ag(I)-malonamide complex. Molecules 23:888. https://doi.org/10.3390/molecules23040888
Funding
The authors acknowledge funding from CAPES (Coordination of Improvement of Higher Education Personnel, Brazil, Funding Code 001 CAPES) and the PROPESQ/Federal University of Tocantins (Edital para tradução de artigos científicos da Universidade Federal do Tocantins, PROPESQ/UFT). The Center for Computational Engineering and Sciences (financial support from FAPESP Fundação de Amparo à Pesquisa, Grant 2013/08293–7 and Grant 2017/11485–6) and the National Center for High Performance Processing (Centro Nacional de Processamento de Alto Desempenho – CENAPAD) in São Paulo for computational resources.
Author information
Authors and Affiliations
Contributions
Sílvio Quintino de Aguiar Filho: Conceptualization, Methodology, Validation, Formal analysis. Adão Marcos Ferreira Costa: Visualization, Software, Formal analysis. Anna Karla dos Santos Pereira: Writing — review and editing, Visualization, Software. Grasiele Soares Cavallini: Writing — original draft, Writing — review and editing, Conceptualization, Methodology. Douglas Henrique Pereira: Writing — original draft, Writing — review and editing, Conceptualization, Methodology, Formal analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection VIII Symposium on Electronic Structure and Molecular Dynamics – VIII SeedMol
Rights and permissions
About this article
Cite this article
de Aguiar Filho, S.Q., Costa, A.M.F., dos Santos Pereira, A.K. et al. Interaction of glyphosate in matrices of cellulose and diethylaminoethyl cellulose biopolymers: theoretical viewpoint of the adsorption process. J Mol Model 27, 272 (2021). https://doi.org/10.1007/s00894-021-04894-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04894-y