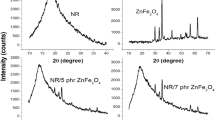
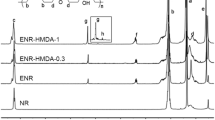
Abstract
Rubber compounds contain several additives besides the elastomer matrix, such as fillers, curing agents, activators, antioxidants, among others. However, some of these additives causes a negative impact on the environment, e.g., zinc oxide commonly used as activator in the vulcanization process. Thus, in this study, ecofriendly synthesized magnesium oxide was evaluated as substitute to ZnO in a standard nitrile rubber composition. Additionally, commercial magnesium oxide was also included in study to be used as a reference. All rubber compounds were evaluated in terms of rheometric (minimum and maximum torques, curing times), rheological (dynamic moduli), and mechanical (tear resistance, tensile resistance, hardness, and stress relaxation) properties and crosslink density. The marching-modulus was more noticeable when MgO and green-MgO was employed as activator. The vulcanization of NBR with MgO and green-MgO resulted in rubber materials with higher crosslink density, higher elastic moduli, lower phase angle δ, and lower stress relaxation than ZnO composites. Besides that, no significant difference was observed for the mechanical performance regarding tear strength, tensile strength, and hardness.






Similar content being viewed by others
References
Lou W, Zhang W, Wang H, Jin T, Liu X (2018) Influence of hydraulic oil on degradation behavior of nitrile rubber O-rings at elevated temperature. Eng Fail Anal 92:1–11. https://doi.org/10.1016/j.engfailanal.2018.05.006
Wei X-q, Wu H-l, Zhang L-w, Zhang S-y, Xiao Y, Luo T-y (2018) Failure analysis of nitrile rubber o-rings static sealing for packaging barrel. J Fail Anal Prev 18:628–634. https://doi.org/10.1007/s11668-018-0451-3
Joseph AM, George B, Madhusoodanan KN, Alex R (2015) Current status of sulphur vulcanization and devulcanization chemistry: process of vulcanization. Rubber Sci 28:82–121
Mostoni M, Credico D (2019) Zinc-Based curing activators: new trends for reducing zinc content in rubber vulcanization process. Catalysts 9:664. https://doi.org/10.3390/catal9080664
Heideman G, Noordermeer JWM, Datta RN, van Baarle B (2006) Various ways to reduce zinc oxide levels in s-sbr rubber compounds. Macromol Symp 245–246:657–667. https://doi.org/10.1002/masy.200651393
Bodar CWM, Pronk MEJ, Sijm DTHM (2005) The european union risk assessment on zinc and zinc compounds: the process and the facts. Integr Environ Assess Manag 1:301–319
Sahoo S, Maiti M, Ganguly A, Jacob George J, Bhowmick AK (2007) Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber. J Appl Polym Sci 105:2407–2415. https://doi.org/10.1002/app.26296
Przybyszewska M, Zaborski M (2009) The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomer. Express Polym Lett 3:542–552. https://doi.org/10.3144/expresspolymlett.2009.68
Roy K, Alam MN, Mandal SK, Debnath SC (2014) Sol–gel derived nano zinc oxide for the reduction of zinc oxide level in natural rubber compounds. J Sol-Gel Sci Technol 70:378–384. https://doi.org/10.1007/s10971-014-3293-9
Zanchet A, de Sousa FDB, Crespo JS, Scuracchio CH (2018) Activator from sugar cane as a green alternative to conventional vulcanization additives. J Clean Prod 174:437–446. https://doi.org/10.1016/j.jclepro.2017.10.329
Zanchet A, Demori R, de Sousa FDB, Ornaghi HL Jr, Schiavo LSA, Scuracchio CH (2019) Sugar cane as an alternative green activator to conventional vulcanization additives in natural rubber compounds: Thermal degradation study. J Clean Prod 207:248–260. https://doi.org/10.1016/j.jclepro.2018.09.203
Guzmán M, Reyes G, Agulló N, Borrós S (2011) Synthesis of Zn/Mg oxide nanoparticles and its influence on sulfur vulcanization. J Appl Polym Sci 119:2048–2057. https://doi.org/10.1002/app.32885
Guzmán M, Vega B, Agulló N, Giese U, Borrós S (2012) Zinc oxide versus magnesium oxide revisited. part 1. Rubber Chem Technol 85:38–55. https://doi.org/10.5254/1.3672428
Guzmán M, Vega B, Agulló N, Borrós S (2012) Zinc oxide versus magnesium oxide revisited. part 2. Rubber Chem Technol 85:56–67. https://doi.org/10.5254/1.3672429
Roy K, Alam MN, Mandal SK, Debnath SC (2015) Preparation of zinc-oxide-free natural rubber nanocomposites using nanostructured magnesium oxide as cure activator. J Appl Polym Sci 132:42705. https://doi.org/10.1002/app.42705
Jeevanandam J, Chan YS, Danquah MK (2017) Calcination-dependent morphology transformation of sol-gel- synthesized MgO nanoparticles. Chemistry Select 2:10393–10404. https://doi.org/10.1002/slct.201701911
Sharmila G, Muthukumaran C, Sangeetha E, Saraswathi H, Soundarya S, Kumar NM (2019) Green fabrication, characterization of Pisonia alba leaf extract derived MgO nanoparticles and its biological applications. Nano-Struct Nano-Obj 20:100380. https://doi.org/10.1016/j.nanoso.2019.100380
Nijalingappa TB, Veeraiah MK, Basavaraj RB, Darshan GP, Sharma SC, Nagabhushana H (2019) Antimicrobial properties of green synthesis of MgO micro architectures via Limonia acidissima fruit extract. Biocatal Agric Biotechnol 18:100991. https://doi.org/10.1016/j.bcab.2019.01.029
Raveesha HR, Nayana S, Vasudha DR, Begum JPS, Pratibha S, Ravikumara CR, Dhananjaya N (2019) The electrochemical behavior, antifungal and cytotoxic activities of phytofabricated MgO nanoparticles using Withania somnifera leaf extract. J Sci: Adv Mater Devices 4:57–65. https://doi.org/10.1016/j.jsamd.2019.01.003
Suresh J, Pradheesh G, Alexramani V, Sundrarajan M, Hong SI (2018) Green synthesis and characterization of hexagonal shaped MgO nanoparticles using insulin plant ( Costus pictus D. Don) leave extract and its antimicrobial as well as anticancer activity. Adv Powder Technol 29:1685–1694. https://doi.org/10.1016/j.apt.2018.04.003
Jhansi K, Jayarambabu N, Reddy KP, Reddy NM, Suvarna RP, Rao KV, Kumar VR, Rajendar V (2017) Biosynthesis of MgO nanoparticles using mushroom extract: effect on peanut (Arachis hypogaea L.) seed germination. Biotech 7:263. https://doi.org/10.1007/s13205-017-0894-3
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv 31:346–356. https://doi.org/10.1016/j.biotechadv.2013.01.003
Senthilkumar SR, Sivakumar T (2014) Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int J Pharm Pharm Sci 6:461–465
Peralta-Videa JR, Huang Y, Parsons JG, Zhao L, Lopez-Moreno L, Hernandez-Viezcas JA, Gardea-Torresdey JL (2016) Plant-based green synthesis of metallic nanoparticles: scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol Environ Eng. https://doi.org/10.1007/s41204-016-0004-5
Yasin SA, Abbas JA, Saeed IA, Ahmed IH (2019) The application of green synthesis of metal oxide nanoparticles embedded in polyethylene terephthalate nanofibers in the study of the photocatalytic degradation of methylene blue. Polym Bull 77:3473–3484. https://doi.org/10.1007/s00289-019-02919-4
Silva AA, Rocha EBD, Furtado CRG, Sousa AMF, Carvalho NMF (2020) Magnesium oxide biosynthesized with Camellia sinensis extract as activator in nitrile rubber vulcanization. Polym Bull. https://doi.org/10.1007/s00289-020-03430-x
Pechurai W, Sahakaro K, Nakason C (2009) Influence of phenolic curative on crosslink density and other related properties of dynamically cured NR/HDPE blends. J Appl Polym Sci 113:1232–1240. https://doi.org/10.1002/app.30036
Mitra S, Ghanbari-Siahkali A, Almdal K (2006) A novel method for monitoring chemical degradation of crosslinked rubber by stress relaxation under tension. Polym Degrad Stab 91:2520–2526. https://doi.org/10.1016/j.polymdegradstab.2006.03.002
Chakraborty SK, De SK (1983) Effect of curing systems on polymer-filler interaction, technical properties and fracture mode of carboxylated nitrile rubber. Polymer 24:1055–1062. https://doi.org/10.1016/0032-3861(83)90159-3
Jin J, Noordermeer JWM, Dierkes WK, Blume A (2020) The effect of silanization temperature and time on the marching modulus of silica-filled tire tread compounds. Polymers (Basel) 12:209. https://doi.org/10.3390/polym12010209
Jin J, Noordermeer JWM, Dierkes WK, Blume A (2020) The origin of marching modulus of silica-filled tire tread compounds. Rubber Chem Technol 93:378–394. https://doi.org/10.5254/rct.19.80453
Fröhlich J, Niedermeier W, Luginsland HD (2005) The effect of filler–filler and filler–elastomer interaction on rubber reinforcement. Compos A Appl Sci Manuf 36:449–460. https://doi.org/10.1016/j.compositesa.2004.10.004
Litvinov VM, Orza RA, Klüppel M, van Duin M, Magusin PCMM (2011) Rubber-filler interactions and network structure in relation to stress-strain behavior of vulcanized, carbon black filled EPDM. Macromolecules 44:4887–4900. https://doi.org/10.1021/ma2007255
Müllner HW, Jäger A, Aigner EG, Eberhardsteiner J (2008) Experimental identification of viscoelastic properties of rubber compounds by means of torsional rheometry. Meccanica 43:327–337. https://doi.org/10.1007/s11012-007-9097-z
Shangguan Y, Yang J, Zheng Q (2017) Rheology of nitrile rubber with hybrid crosslinked network composed of covalent bonding and hydrogen bonding. RSC Adv 7:15978–15985. https://doi.org/10.1039/c7ra01106g
Chandra CJ, Bipinbal PK, Sunil KN (2017) Viscoelastic behaviour of silica filled natural rubber composites – correlation of shear with elongational testing. Polym Testing 60:187–197. https://doi.org/10.1016/j.polymertesting.2017.03.023
Syed IH, Stratmann P, Hempel G, Klüppel M, Saalwächter K (2016) Entanglements, defects, and inhomogeneities in nitrile butadiene rubbers: macroscopic versus microscopic properties. Macromolecules 49:9004–9016. https://doi.org/10.1021/acs.macromol.6b01802
Abu-Abdeen M (2010) Single and double-step stress relaxation and constitutive modeling of viscoelastic behavior of swelled and un-swelled natural rubber loaded with carbon black. Mater Des 31:2078–2084. https://doi.org/10.1016/j.matdes.2009.10.006
Karrab M, Mohammadian-Gezaz S (2011) The effects of carbon black-based interactions on the linear and non-linear viscoelasticity of uncured and cured SBR compounds. Iran Polym J 20:15–27
Maria HJ, Lyczko N, Nzihou A, Joseph K, Mathew C, Thomas S (2014) Stress relaxation behavior of organically modified montmorillonite filled natural rubber/nitrile rubber nanocomposites. Appl Clay Sci 87:120–128. https://doi.org/10.1016/j.clay.2013.10.019
da Rocha EBD, Linhares FN, Gabriel CFS, de Sousa AMF, Furtado CRG (2018) Stress relaxation of nitrile rubber composites filled with a hybrid metakaolin/carbon black filler under tensile and compressive forces. Appl Clay Sci 151:181–188. https://doi.org/10.1016/j.clay.2017.10.008
Acknowledgements
The authors acknowledge the Nitriflex for rubbers donation, the Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPQ (306171/2018-00), the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro–FAPERJ (JCNE 2018: E-26/203.023/2018), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (Financing code 001).
Author information
Authors and Affiliations
Contributions
AAS: Investigation, validation, Writing—original draft, Data curation. EBDR: Investigation related to rubber compounding, Writing—review & editing. FNL: Data curation, discussion of the results, writing, review & editing. CRGF: Supervision, Project administration, Writing—review & editing. AMFS: Conceptualization regarding rubber methodology, Funding acquisition, Resources, Writing—review & editing. NMFC: Conceptualization regarding green synthesis methodology, Funding acquisition, Resources, Writing—review & editing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, A.A., da Rocha, E.B.D., Linhares, F.N. et al. Replacement of ZnO by ecofriendly synthesized MgO in the NBR vulcanization. Polym. Bull. 79, 8535–8549 (2022). https://doi.org/10.1007/s00289-021-03921-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03921-5