Abstract
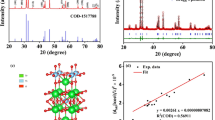
In situ chemical polymerization method was used to synthesize the polyaniline (PANI) and polyaniline cadmium oxide (PANI-CdO) nanocomposites. The morphology and structure of pure PANI and PANI-CdO nanocomposites were characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis, whereas electrical properties were studied by dielectric, electric modulus and a.c conductivity. Various dopant-to-polymer ratios were used to investigate their effect on the characteristics of the synthesized samples. The SEM images exhibited granular as well as flaky structures of PANI and PANI-CdO nanocomposites. The XRD patterns revealed that pure PANI exhibits amorphous nature, while PANI-CdO nanocomposites exhibit polycrystalline nature. The crystallinity and intensity of (XRD) peaks of composite are enhanced by increasing CdO contents. The dielectric measurements show a decrease in dielectric constant, dielectric loss and a decrease in tangent loss with the increase in frequency and nearly constant values at higher frequencies, while the values of dielectric properties increase with the rise in temperature and doping concentration. The electric field modulus was used to analyze the relaxation behavior of the synthesized samples and found to be increased with frequency and decreased with the temperature and CdO concentration. The a.c conductivity was observed to increase with the increase in frequency and temperature for PANI and PANI-CdO composites. The changing behavior of the frequency exponent (S) at various temperatures was analyzed to observe different conduction mechanisms, and a correlated barrier hopping model (CBH) was found to be observed in PANI-CdO composites as well as in pure PANI. The Log a.c conductivity decreases versus the inverse of temperature and with increase in frequency that confirms that the hopping mechanism is the dominant charge transport mechanism.











Similar content being viewed by others
References
Parker ID (1994) Carrier tunneling and device characteristics in polymer light-emitting diodes. J Appl Phys 75:1656–1666. https://doi.org/10.1063/1.356350
Jeon H, Ding J, Nurmikko AV, Xie W, Grillo DC, Kobayashi M, Gunshor RL, Hua GC, Otsuka N (1992) Blue and green diode lasers in ZnSe‐based quantum wells. Appl Phys Lett 60:2045–2047. https://doi.org/10.1063/1.107109
Rolo AG, Vieira LG, Gomes MJM, Ribeiro JL, Belsley MS, Santos MP (1998) Growth and characterisation of cadmium sulphide nanocrystals embedded in silicon dioxide films. Thin Solid Films 312:348–353. https://doi.org/10.1016/S0040-6090(97)00233-2
Manickathai K, Viswanathan SK, Alagar M (2008) Synthesis and characterization of CdO and CdS nanoparticles. Indian J Pure App Phys 46:561–564. https://www.researchgate.net/publication/279548537
Majid A, Afza Z, Mutaza S, Nabi G, Ahmad N (2013) Synthesis and characterization of silver doped cadmium oxide nanoparticles. J Adv Phys 02:116–118. https://doi.org/10.1166/jap.2013.1058
Kondawar S, Mahore R, Dahegaonkar A, Agrawal S (2011) Electrical conductivity of cadmium oxide nanoparticles embedded polyaniline nanocomposites. Adv App Sci Res 2:401–406. https://www.pelagiaresearchlibrary.com
Heidari A, Brown C (2015) Study of composition and morphology of cadmium oxide (Cdo) nanoparticles for eliminating cancer cells. J Nanomed Res 2:1–20. https://doi.org/10.15406/jnmr.2015.02.00042
Ferro R, Rodriguez JA (2000) Influence of F-doping on the transmittance and electron affinity of CdO thin films suitable for solar cells technology. Energy Mater Sol Cells 64:363–370. https://doi.org/10.1016/S0927-0248(00)00228-2
Li J, Ni YH, Liu J, Hong J (2009) Preparation, conversion, and comparison of the photocatalytic property of Cd(OH)2, CdO, CdS and CdSe. J Phys Chem Solids 70:1285–1289. https://doi.org/10.1016/j.jpcs.2009.07.014
Liu Y, Zhang YC, Xu XF (2009) Hydrothermal synthesis and photocatalytic activity of CdO2 nanocrystals. J Hazard Mater 163:1310–1314. https://doi.org/10.1016/j.jhazmat.2008.07.101
Lu HB, Liao L, Li JC, Wang DF, He H, Fu Q, Xu L, Tian Y (2006) High surface-to-volume ratio ZnO microberets: low temperature synthesis, characterization, and photoluminescence. J Phys Chem B 110:23211–23214. https://doi.org/10.1021/jp064079r
Laranjeira JMG, Khoury HJ, de Azevedo WM, da Silva Jr EF, da Vasconcelos EA (2002) A silicon-polymer heterostructure for sensor applications. Braz J Phys 32:421–423. https://doi.org/10.1590/S0103-97332002000200050
Kalaycioglu E, Akbulut U, Toppare L (1996) Conducting composites of polypyrrole with polytetramethylbisphenol a carbonate. J Appl Polym Sci 61:1067–1075. https://doi.org/10.1002/(SICI)1097-4628(19960815)61:7<1067::AID-APP1>3.0.CO;2-K
Koezuka H, Tsumura A (1989) Field-effect transistor utilizing conducting polymers. Synth Met 28:C-753–760. https://doi.org/10.1016/0379-6779(89)90600-0
Gustafsson G, Treacy GM, Cao Y, Klavetter F, Colaneri N, Heeger AJ (1993) The “plastic” led: A flexible light-emitting device using a polyaniline transparent electrode. Synth Met 57:4123–4127. https://doi.org/10.1016/0379-6779(93)90568-H
Chiang JC, Macdiarmid AG (1986) ‘Polyaniline’: Protonic acid doping of the emeraldine form to the metallic regime. Synth Met 13:193–205. https://doi.org/10.1016/0379-6779(86)90070-6
Gustafsson G, Cao Y, Treacy GM, Klavetter F, Colaneri N, Heeger AJ (1992) Flexible light-Emitting diodes from soluble conducting polymers. Nature 357:477–479. https://doi.org/10.1038/357477a0
Sailor MJ, Ginsburg EJ, Gorman CB, Kumar A, Grubbs RH, Lewis NS (1990) Thin films of n-Si/Poly-(CH3)3Si-Cyclooctatetraene: conducting-polymer solar cells and layered structures. Science 249:1146–1149. https://doi.org/10.1126/science.249.4973.1146
Li L, Jiang J, Xu F (2007) Synthesis and ferrimagnetic properties of novel Sm-substituted LiNi ferrite–polyaniline nanocomposite. Mater Lett 61:1091–1096. https://doi.org/10.1016/j.matlet.2006.06.061
Ayad MM, Zaki EA (2008) Doping of polyaniline films with organic sulfonic acids in aqueous media and the effect of water on these doped films. Eur Polymer J 44:3741–3747. https://doi.org/10.1016/j.eurpolymj.2008.08.012
Chung SF, Wen TC, Gopalan A (2005) Influence of dopant size on the junction properties of polyaniline. Mater Sci Eng B 116:125–130. https://doi.org/10.1016/j.mseb.2004.09.023
Long Y, Chen Z, Wang N, Li J, Wan M (2004) Electronic transport in PANI-CSA/PANI-DBSA polyblends. Phys B: Conden Matter 344:82–87. https://doi.org/10.1016/j.physb.2003.09.245
Roy AS, Anilkumar KR, Ambika Prasad MVN (2012) Studies of AC conductivity and dielectric relaxation behavior of CdO-doped nanometric polyaniline. J Appl Polymer Sci 123:1928–1934. https://doi.org/10.1002/app.34696
Xu JC, Liu WM, Li HL (2005) Titanium dioxide doped polyaniline. Mater Sci Eng C 25:444–447. https://doi.org/10.1016/j.msec.2004.11.003
Mo TC, Wang HW, Chen SY, Yeh YC (2008) Synthesis and dielectric properties of polyaniline/titanium dioxide nanocomposites. Ceram Int 34:1767–1771. https://doi.org/10.1016/j.ceramint.2007.06.002
Shi L, Wang X, Lu L, Yang X, Wu X (2009) Preparation of TiO2/polyaniline nanocomposite from a lyotropic liquid crystalline solution. Synth Met 159:2525–2529. https://doi.org/10.1016/j.synthmet.2009.08.056
Jia W, Segal E, Kornemandel D, Lamhot Y, Narkis M, Siegmann A (2002) Polyaniline–DBSA/organophilic clay nanocomposites: synthesis and characterization. Synth Met 128:115–120. https://doi.org/10.1016/S0379-6779(01)00672-5
Liu P (2008) Preparation and characterization of conducting polyaniline/silica nanosheet composites. Mater Sci 12:9–13. https://doi.org/10.1016/J.COSSMS.2009.01.001
Jing S, Xing S, Yu L, Wu Y, Zhao C (2007) Synthesis and characterization of Ag/polyaniline core–shell nanocomposites based on silver nanoparticles colloid. Mater Lett 61:2794–2797. https://doi.org/10.1016/j.matlet.2006.10.032
Khanna PK, Singh N, Charan S, Visawanath AK (2005) Synthesis of Ag/polyaniline nanocomposite via an in situ photo-redox mechanism. Mater Chem Phys 92:214–219. https://doi.org/10.1016/j.matchemphys.2005.01.011
Kim BH, Jung JH, Kim JW, Choi HJ, Joo J (2001) Physical characterization of polyaniline-Na+-montmorillonite nanocomposite intercalated by emulsion polymerization. Synth Met 117:115–118. https://doi.org/10.1016/S0379-6779(00)00549-X
He Y (2005) Synthesis of polyaniline/nano-CeO2 composite microspheres via a solid-stabilized emulsion route. Mater Chem Phys 92:134–137. https://doi.org/10.1016/j.matchemphys.2005.01.033
Xue W, Fang K, Qiu H, Li J, Mao W (2006) Electrical and magnetic properties of the Fe3O4–polyaniline nanocomposite pellets containing DBSA-doped polyaniline and HCl-doped polyaniline with Fe3O4 nanoparticles. Synth Met 156:506–509. https://doi.org/10.1016/j.synthmet.2005.06.021
Olad A, Barati M, Shirmohammadi H (2011) Conductivity and anticorrosion performance of polyaniline/zinc composites: investigation of zinc particle size and distribution effect. Prog Org Coat 72:599–604. https://doi.org/10.1016/j.porgcoat.2011.06.022
Zhang X, Ji L, Zhang S, Yang W (2007) Synthesis of a novel polyaniline-intercalated layered manganese oxide nanocomposite as electrode material for electrochemical capacitor. J Power Sources 173:1017–1023. https://doi.org/10.1016/j.jpowsour.2007.08.083
Shakoor A, Anwar H, Rizvi TZ (2008) Structural and electrical properties of doped polypyrrole and its composite with montmorillonite clay. J Compos Mater 42:2101–2109. https://doi.org/10.1134/S0965545X1304010X
Zargar RA, Chackarabarti S, Arora M, Hafiz AK (2016) Synthesis, characterization and interpretation of screen-printed nanocrystalline CdO thick film for optoelectronic applications. Int Nano Lett 6:99–104. https://doi.org/10.1007/s40089-015-0172-5
Xingwei L, Wang G, Xiaoxuan L, Dongming L (2004) Surface properties of polyaniline/nano-TiO2 composites. Appl Surf Sci 229:395–401. https://doi.org/10.1016/j.apsusc.2004.02.022
Zheng L, Xu Y, Jin D, Xie Y (2011) Polyaniline-intercalated molybdenum oxide nanocomposites: simultaneous synthesis and their enhanced application for supercapacitor. Chem Asian J 6:1505–1514. https://doi.org/10.1002/asia.201000770
Jaidev RI, Jafri AK, Mishra SR (2011) Polyaniline–MnO2 nanotube hybrid nanocomposite as supercapacitor electrode material in acidic electrolyte. J Mater Chem 21:17601–17605. https://doi.org/10.1039/C1JM13191E
Bragg WL (1913) The diffraction of short electromagnetic waves by a crystal. Proc Cambridge Philos Soc 17:43–57
Patterson AL (1939) The scherrer formula for X-ray particle size determination. Phys Rev American Phys Soc 56:978–982. https://link.aps.org/doi/10.1103/PhysRev.56.978
Ahmad Z (2012) Polymer dielectric materials. In: Dielectric Material. University Sains Malaysia, pp 3–26. https://doi.org/10.5772/50638
Psarras GC (2006) Hopping conductivity in polymer matrix–metal particles composites. Compos Part A Appl Sci Manuf 37:1545–1553. https://doi.org/10.1016/j.compositesa.2005.11.004
Wang L, Dang ZM (2005) Carbon nanotube composites with high dielectric constant at low percolation threshold. Appl Phys Lett 87(1–3):042903. https://doi.org/10.1063/1.1996842
Pinto NJ, Sinha GP, Aliev FM (1998) Frequency-dependent conductivity and dielectric permittivity of emeraldine base and weakly doped poly(o-toluidine). Synth Met 94:199–203. https://doi.org/10.1016/S0379-6779(98)00003-4
Patankar KK, Dombale PD, Mathe VL, Patil SA, Patil RN (2001) AC conductivity and magnetoelectric effect in MnFe1.8Cr0.2O4–BaTiO3 composites. Mater Sci Eng B 87:53–58. https://doi.org/10.1016/S0921-5107(01)00695-X
Idrees M, Nadeem M, Atif M, Siddique M, Mehmood M, Hassan MM (2011) Origin of colossal dielectric response in LaFeO3. Acta Mater 59:1338–1345. https://doi.org/10.1016/j.actamat.2010.10.066
Vishwanathan B, Murthy VRK (1990) Ferrite materials: science and technology. In: Ferrite materials. Narosa Publishing House, New Delhi, p 6
Maxwell JC (1892) A treatise on electricity and magnetism, 3rd edn. Clarendon press, Oxford
Wagner KW (1914) Explanation of the dielectric after-effect processes on the basis of Maxwell's ideas. Arch Elektrotech 2:371–387. https://doi.org/10.1007/BF01657322
Wagner KW (1913) The distribution of relaxation times in typical dielectrics. Ann Phys 40:817–855
Himansh AK, Ray DK, Sinha TP (2005) Ac conductivity of conducting polymer prepared with the use of water soluble support polymer. Indian J Phys 79:1049–1052. https://192.168.1.41:8080/xmlui/handle/123456789/2292
Matteeva ES (1996) Residual water as a factor influencing the electrical properties of polyaniline. The role of hydrogen bonding of the polymer with solvent molecules in the formation of a conductive polymeric network. Synth Met 79:127–139. https://doi.org/10.1016/0379-6779(96)80180-9
Bhat S, Khosa SK, Kotru PN, Tandon RP (1995) Dielectric studies of lanthanum heptamolybdate crystals grown from gels. Mater Sci Eng B 309:7–11. https://doi.org/10.1016/0921-5107(94)01129-x
Mantas PQ (1999) Dielectric response of materials: extension to the debye model. J Eur Ceram Soc 19:2079–2086. https://doi.org/10.1016/S0955-2219(98)00273-8
Tonks DL, Silver RN (1982) Small-polaron models for the hydrogen-concentration dependence of hydrogen diffusion in Nb. Phys Rev B 26(12):6455–6469. https://doi.org/10.1103/PhysRevB.26.6455
Ghosh A (1990) ac conduction in iron bismuthate glassy semiconductors. Phys Rev B 42(2):1388–1393. https://doi.org/10.1103/PhysRevB.42.1388
Pike GE (1972) ac conductivity of scandium oxide and a new hopping model for conductivity. Phys Rev B 6(4):1572–1580. https://doi.org/10.1103/PhysRevB.6.1572
Jonscher AK (1977) The ‘universal’ dielectric response. Nature 267:673–679. https://doi.org/10.1038/267673a0
Long AR (1982) Frequency-dependent loss in amorphous semiconductors. Adv Phys 31:553–637. https://doi.org/10.1080/00018738200101418
Farid AM, Atyia HE, Hegab NA (2005) AC conductivity and dielectric properties of Sb2Te3 thin films. Vacuum 80:284–294. https://doi.org/10.1016/j.vacuum.2005.05.003
Elliott SR (1978) Temperature dependence of a.c. conductivity of chalcogenide glasses. Philos Mag B 37:553–560. https://doi.org/10.1080/01418637808226448
Street RA, Mott NF (1975) States in the gap in glassy semiconductors. Phys Rev Lett 35:1293–1296. https://doi.org/10.1103/PhysRevLett.35.1293
Shimakawa K (1982) On the temperature dependence of a.c. conduction in chalcogenide glasses. Philos Mag B 46:123–135. https://doi.org/10.1080/13642818208246429
Rockstad HK (1969) Evidence for hopping conduction in amorphous chalcogenide films. Solid State Commun 7:1507–1509. https://doi.org/10.1016/0038-1098(69)90031-3
Rockstad HK (1971) Comments on the a.c. conductivity of amorphous chalcogenides. Solid State Commun 9:2233–2237. https://doi.org/10.1016/0038-1098(71)90637-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anwar, N., Shakoor, A., Niaz, N.A. et al. Investigation of dielectric relaxation behavior, electric modulus and a.c conductivity of low doped polyaniline cadmium oxide (PANI-CdO) nanocomposites. Polym. Bull. 79, 6581–6600 (2022). https://doi.org/10.1007/s00289-021-03766-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03766-y