Abstract
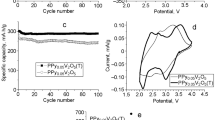
A hybrid hydrogel based on polypyrrole and hydrothermally prepared α-Fe2O3 nanoparticles was synthesized via in situ chemical polymerization of pyrrole using sodium trimetaphosphate as a crosslinker. Wide-angle X-ray diffraction study confirmed the presence of α-Fe2O3 in the prepared material. Mapping of the elemental composition using energy dispersive X-ray spectroscopy showed the uniform distribution of the inorganic particles inside the polypyrrole matrix. The effect of α-Fe2O3 on the structure of the hybrid hydrogel and on the mechanism of charge storage was studied with scanning electron microscopy, cyclic voltammetry, galvanostatic charge–discharge and impedance spectroscopy. The specific capacitance was found to increase from 250 F g−1 for the polypyrrole hydrogel up to 509 F g−1 for the α-Fe2O3-doped hydrogel at the current density of 0.2 A g−1. The hematite incorporation also affected the morphology of the hydrogel leading to a slight increase in the double-layer capacitance accompanied with a strong increase in the pseudocapacitance: from 239 F g−1 up to 486 F g−1. The initial polypyrrole hydrogel and the hybrid hydrogel demonstrated a capacitance retention of about 75% and 79% after 3000 charge–discharge cycles at the current density of 4 A g−1, respectively.








Similar content being viewed by others
References
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828. https://doi.org/10.1039/c1cs15060j
Pérez-Madrigal MM, Estrany F, Armelin E et al (2016) Towards sustainable solid-state supercapacitors: electroactive conducting polymers combined with biohydrogels. J Mater Chem A 4:1792–1805. https://doi.org/10.1039/c5ta08680a
Dubal DP, Chodankar NR, Kim DH, Gomez-Romero P (2018) Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem Soc Rev 47:2065–2129. https://doi.org/10.1039/c7cs00505a
Meng Q, Cai K, Chen Y, Chen L (2017) Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 36:268–285. https://doi.org/10.1016/j.nanoen.2017.04.040
Smirnov MA, Sokolova MP, Geydt P et al (2017) Dual doped electroactive hydrogelic fibrous mat with high areal capacitance. Mater Lett 199:192–195. https://doi.org/10.1016/j.matlet.2017.04.083
Armelin E, Pérez-Madrigal MM, Alemán C, Díaz DD (2016) Current status and challenges of biohydrogels for applications as supercapacitors and secondary batteries. J Mater Chem A 4:8952–8968. https://doi.org/10.1039/c6ta01846g
Patil DS, Pawar SA, Devan RS et al (2013) Electrochemical supercapacitor electrode material based on polyacrylic acid/polypyrrole/silver composite. Electrochim Acta 105:569–577. https://doi.org/10.1016/j.electacta.2013.05.022
Davoglio RA, Biaggio SR, Bocchi N, Rocha-Filho RC (2013) Flexible and high surface area composites of carbon fiber, polypyrrole, and poly(DMcT) for supercapacitor electrodes. Electrochim Acta 93:93–100. https://doi.org/10.1016/j.electacta.2013.01.062
Wang Z, Tammela P, Huo J et al (2016) Solution-processed poly (3,4-ethylenedioxythiophene) nanocomposite paper electrodes for high-capacitance flexible supercapacitors. J Mater Chem A 4:1714–1722. https://doi.org/10.1039/C5TA10122K
Wang JG, Yang Y, Huang ZH, Kang F (2014) MnO2/polypyrrole nanotubular composites: reactive template synthesis, characterization and application as superior electrode materials for high-performance supercapacitors. Electrochim Acta 130:642–649. https://doi.org/10.1016/j.electacta.2014.03.082
Lu Q, Zhou Y (2011) Synthesis of mesoporous polythiophene/MnO2 nanocomposite and its enhanced pseudocapacitive properties. J Power Sources 196:4088–4094. https://doi.org/10.1016/j.jpowsour.2010.12.059
Ren S, Ma S, Yang Y et al (2015) Hydrothermal synthesis of Fe2O3/polypyrrole/graphene oxide composites as highly efficient electrocatalysts for oxygen reduction reaction in alkaline electrolyt. Electrochim Acta 178:179–189. https://doi.org/10.1016/j.electacta.2015.07.181
Arjomandi J, Lee JY, Movafagh R et al (2018) Polyaniline/aluminum and iron oxide nanocomposites supercapacitor electrodes with high specific capacitance and surface area. J Electroanal Chem 810:100–108. https://doi.org/10.1016/j.jelechem.2017.12.086
Xie A, Tao F, Li T et al (2018) Graphene-cerium oxide/porous polyaniline composite as a novel electrode material for supercapacitor. Electrochim Acta 261:314–322. https://doi.org/10.1016/j.electacta.2017.12.165
Kumar AM, Babu RS, Ramakrishna S, de Barros ALF (2017) Electrochemical synthesis and surface protection of polypyrrole-CeO2 nanocomposite coatings on AA2024 alloy. Synth Met 234:18–28. https://doi.org/10.1016/j.synthmet.2017.10.003
Hjiri M, Aida MS, Neri G (2019) NO2 selective sensor based on α-Fe2O3 nanoparticles synthesized via hydrothermal technique. Sensors 19:1–11. https://doi.org/10.3390/s19010167
Chaudhari NK, Chaudhari S, Yu JS (2014) Cube-like α-Fe2O3 supported on ordered multimodal porous carbon as high performance electrode material for supercapacitors. Chemsuschem 7:3102–3111. https://doi.org/10.1002/cssc.201402526
Shivakumara S, Penki TR (2014) Preparation and electrochemical performance of porous hematite ( α-Fe2O3) nanostructures as supercapacitor electrode material. J Solid State Electrochem 18:1057–1066. https://doi.org/10.1007/s10008-013-2355-1
Kamali KZ, Alagarsamy P, Huang NM et al (2014) Hematite nanoparticles-modified electrode based electrochemical sensing platform for dopamine. Sci World J 2014:1–13. https://doi.org/10.1155/2014/396135
Li B, Sun Q, Fan H et al (2018) Morphology-controlled synthesis of hematite nanocrystals and their optical, magnetic and electrochemical performance. Nanomaterials 8:1–12. https://doi.org/10.3390/nano8010041
Zhu M, Wang Y, Meng D et al (2012) Hydrothermal synthesis of hematite nanoparticles and their electrochemical properties. J Phys Chem C 116:16276–16285. https://doi.org/10.1021/jp304041m
Zhang X, Niu Y, Li Y et al (2013) Synthesis, optical and magnetic properties of α-Fe2O3 nanoparticles with various shapes. Mater Lett 99:111–114. https://doi.org/10.1016/j.matlet.2013.02.070
Lin M, Tng L, Lim T et al (2014) Hydrothermal synthesis of octadecahedral hematite (α-Fe2O3) nanoparticles: an epitaxial growth from goethite (α-FeOOH). J Phys Chem C 118:10903–10910. https://doi.org/10.1021/jp502087h
Gangopadhyay R, De A (2000) Conducting polymer nanocomposites: a brief overview. Chem Mater 12:608–622. https://doi.org/10.1021/cm990537f
Fu S, Ma L, Gan M et al (2017) 3D reduced graphene oxide/MnO2/polyaniline composite for high-performance supercapacitor. J Mater Sci Mater Electron 28:3621–3629. https://doi.org/10.1007/s10854-016-5964-5
Mu B, Zhang W, Shao S, Wang A (2014) Glycol assisted synthesis of graphene-MnO2-polyaniline ternary composites for high performance supercapacitor electrodes. Phys Chem Chem Phys 16:7872–7880. https://doi.org/10.1039/c4cp00280f
Benhaddad L, Bernard MC, Deslouis C et al (2013) Chemical synthesis of hollow sea urchin like nanostructured polypyrrole particles through a core-shell redox mechanism using a MnO2 powder as oxidizing agent and sacrificial nanostructured template. Synth Met 175:192–199. https://doi.org/10.1016/j.synthmet.2013.05.010
He Y, Du S, Li H et al (2016) MnO2/polyaniline hybrid nanostructures on carbon cloth for supercapacitor electrodes. J Solid State Electrochem 20:1459–1467. https://doi.org/10.1007/s10008-016-3162-2
Wang H, Wang X, Peng C et al (2015) Preparation and the electrochemical performance of MnO2/PANI@CNT composite for supercapacitors. J Nanosci Nanotechnol 15:709–714. https://doi.org/10.1166/jnn.2015.9166
Smirnov MA, Sokolova MP, Bobrova NV et al (2016) Capacitance properties and structure of electroconducting hydrogels based on copoly(aniline - P-phenylenediamine) and polyacrylamide. J Power Sources 304:102–110. https://doi.org/10.1016/j.jpowsour.2015.11.035
Wang Z, Tammela P, Zhang P et al (2014) Freestanding nanocellulose-composite fibre reinforced 3D polypyrrole electrodes for energy storage applications. Nanoscale 6:13068–13075. https://doi.org/10.1039/c4nr04642k
Xu D, Xiao X, Cai J et al (2015) Highly rate and cycling stable electrode materials constructed from polyaniline/cellulose nanoporous microspheres. J Mater Chem A 3:16424–16429. https://doi.org/10.1039/c5ta03917g
Yang C, Zhang P, Nautiyal A et al (2019) Tunable three-dimensional nanostructured conductive polymer hydrogels for energy-storage applications. ACS Appl Mater Interfaces 11:4258–4267. https://doi.org/10.1021/acsami.8b19180
Heydari H, Gholivand MB (2016) An all-solid-state asymmetric device based on a polyaniline hydrogel for a high energy flexible supercapacitor. New J Chem 41:237–244. https://doi.org/10.1039/C6NJ02266A
Ding Q, Xu X, Yue Y et al (2018) Nanocellulose-Mediated Electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl Mater Interfaces 10:27987–28002. https://doi.org/10.1021/acsami.8b09656
Zang L, Liu Q, Qiu J et al (2017) Design and fabrication of an all-solid-state polymer supercapacitor with highly mechanical flexibility based on polypyrrole hydrogel. ACS Appl Mater Interfaces 9:33941–33947. https://doi.org/10.1021/acsami.7b10321
Li Y, Zhang H, Ni S, Xiao H (2018) In situ synthesis of conductive nanocrystal cellulose/polypyrrole composite hydrogel based on semi-interpenetrating network. Mater Lett 232:175–178. https://doi.org/10.1016/j.matlet.2018.08.115
Smirnov MA, Bobrova NV, Dmitriev IY et al (2011) Electroactive hydrogels based on poly(acrylic acid) and polypyrrole. Polym Sci - Ser A 53:67–74. https://doi.org/10.1134/S0965545X11010068
Access O, Ding H, Zhong M et al (2014) Biologically derived soft conducting hydrogels using heparin-doped polymer networks. ACS Nano 8:4348–4357. https://doi.org/10.1021/nn406019m
Dai T, Tang R, Yue X et al (2015) Capacitance performances of supramolecular hydrogels based on conducting polymers. Chinese J Polym Sci (English Ed) 33:1018–1027. https://doi.org/10.1007/s10118-015-1647-6
Wang S, Chen X, Shi M et al (2015) Absorption of whey protein isolated (WPI)-stabilized β-Carotene emulsions by oppositely charged oxidized starch microgels. Food Res Int 67:315–322. https://doi.org/10.1016/j.foodres.2014.11.041
Riahi N, Liberelle B, Henry O, De Crescenzo G (2017) Impact of RGD amount in dextran-based hydrogels for cell delivery. Carbohydr Polym 161:219–227. https://doi.org/10.1016/j.carbpol.2017.01.002
Autissier A, Le Visage C, Pouzet C et al (2010) Fabrication of porous polysaccharide-based scaffolds using a combined freeze-drying/cross-linking process. Acta Biomater 6:3640–3648. https://doi.org/10.1016/j.actbio.2010.03.004
Tao Y, Zhang R, Xu W et al (2016) Rheological behavior and microstructure of release-controlled hydrogels based on xanthan gum crosslinked with sodium trimetaphosphate. Food Hydrocoll 52:923–933. https://doi.org/10.1016/j.foodhyd.2015.09.006
Chaouat M, Le Visage C, Baille WE et al (2008) A novel cross-linked poly(vinyl alcohol) (PVA) for vascular grafts. Adv Funct Mater 18:2855–2861. https://doi.org/10.1002/adfm.200701261
Leone G, Bidini A, Lamponi S, Magnani A (2013) States of water, surface and rheological characterisation of a new biohydrogel as articular cartilage substitute. Polym Adv Technol 24:824–833. https://doi.org/10.1002/pat.3150
Leone G, Consumi M, Greco G et al (2011) A PVA/PVP hydrogel for human lens substitution: synthesis, rheological characterization, and in vitro biocompatibility. J Biomed Mater Res Part B Appl Biomater 97:278–288. https://doi.org/10.1002/jbm.b.31813
Ardizzone S, Fregonara G, Trasatti S (1990) “Inner” and “outer” active surface of RuO2 electrodes. Electrochim Acta 35:263–267. https://doi.org/10.1016/0013-4686(90)85068-X
Ma J, Lian J, Duan X et al (2010) α-Fe2O3: Hydrothermal synthesis, magnetic and electrochemical properties. J Phys Chem C 114:10671–10676. https://doi.org/10.1021/jp102243g
Qin W, Yang C, Yi R, Gao G (2011) Hydrothermal synthesis and characterization of single-crystalline α-Fe2O3 nanocubes. J Nanomater 2011:3–8. https://doi.org/10.1155/2011/159259
Wang F, Qin XF, Meng YF et al (2013) Hydrothermal synthesis and characterization of α-Fe2O 3 nanoparticles. Mater Sci Semicond Process 16:802–806. https://doi.org/10.1016/j.mssp.2012.12.029
Chaudhari S, Bhattacharjya D, Yu JS (2013) 1-Dimensional porous α-Fe2O3 nanorods as high performance electrode material for supercapacitors. RSC Adv 3:25120–25128. https://doi.org/10.1039/c3ra44159h
Omastová M, Trchová M, Kovářová J, Stejskal J (2003) Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth Met 138:447–455. https://doi.org/10.1016/S0379-6779(02)00498-8
Murugan R, Mohan S, Bigotto A (1998) FTIR and polarized Raman spectra of acrylamide and polyacrylamide. J Kor Phys Soc 32:505–512
Wang Y, Muramatsu A, Sugimoto T (1998) FTIR analysis of well-defined alpha-Fe2O3 particles. Colloids Surfaces A Physicochem Eng Asp 134:281–297. https://doi.org/10.1016/S0927-7757(97)00102-7
Farahmandjou M, Soflaee F (2015) Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-precipitation method. Phys Chem Res 3:191–196. https://doi.org/10.22036/pcr.2015.9193
Andreeva OA, Burkova LA, Smirnov MA, El’Yashevich GK (2006) Correlation between IR spectra and electric conductivity of polyethylene-polypyrrole composites. Polym Sci Ser B 48:331–334. https://doi.org/10.1134/S1560090406110066
Morris MC, McMurdie HF, Evans EH, et al (1981) Nat. Bur. Stand. (U.S.), Monogr. Washington, p 37
Smirnov MA, Sokolova MP, Bobrova NV et al (2018) Synergistic effect of chitin nanofibers and polyacrylamide on electrochemical performance of their ternary composite with polypyrrole. J Energy Chem 27:843–853. https://doi.org/10.1016/j.jechem.2017.06.002
Arjomandi J, Lee JY, Ahmadi F et al (2017) Spectroelectrochemistry and electrosynthesis of polypyrrole supercapacitor electrodes based on gamma aluminum oxide and gamma iron (III) oxide nanocomposites. Electrochim Acta 251:212–222. https://doi.org/10.1016/j.electacta.2017.08.141
Moyseowicz A, Śliwak A, Miniach E, Gryglewicz G (2017) Polypyrrole/iron oxide/reduced graphene oxide ternary composite as a binderless electrode material with high cyclic stability for supercapacitors. Compos Part B Eng 109:23–29. https://doi.org/10.1016/j.compositesb.2016.10.036
Karaca E, Gökcen D, Pekmez NÖ, Pekmez K (2019) Electrochemical synthesis of PPy composites with nanostructured MnOx, CoOx, NiOx, and FeOx in acetonitrile for supercapacitor applications. Electrochim Acta 305:502–513. https://doi.org/10.1016/j.electacta.2019.03.060
Xu C, Puente-Santiago AR, Rodríguez-Padrón D et al (2019) Controllable Design of Polypyrrole-Iron Oxide Nanocoral Architectures for Supercapacitors with Ultrahigh Cycling Stability. ACS Appl Energy Mater 2:2161–2168. https://doi.org/10.1021/acsaem.8b02167
Yang Z, Qiu A, Ma J, Chen M (2018) Conducting α-Fe2O3 nanorod/polyaniline/CNT gel framework for high performance anodes towards supercapacitors. Compos Sci Technol 156:231–237. https://doi.org/10.1016/j.compscitech.2018.01.012
Wang H, Xu Z, Yi H et al (2014) One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 7:86–96. https://doi.org/10.1016/j.nanoen.2014.04.009
Acknowledgments
The reported study was funded by Russian Foundation for Basic Research (Grant 18-03-01167 a). The experimental work was facilitated by the equipment of the Research Centre for X-ray Diffraction Studies at St. Petersburg State University and of the Engineering Center of the St. Petersburg State Technological Institute (Technical University). The authors are grateful to Dr. I.S. Kuryndin for mechanical measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vorobiov, V.K., Bugrov, A.N., Kasatkin, I.A. et al. Effect of α-Fe2O3 nanoparticles on the mechanism of charge storage in polypyrrole-based hydrogel. Polym. Bull. 78, 2389–2404 (2021). https://doi.org/10.1007/s00289-020-03216-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03216-1