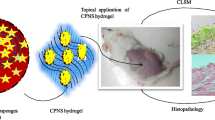
Abstract
The study deals with the formulation of biological macromolecule chitosan-based Tween 80-coated nanoparticles to deliver gallic acid and rutin into the skin for psoriasis treatment. To optimize the nanoformulations for minimum particle size and maximum possible entrapment efficiency (dependent variables), 32 full factorial design was used to optimize the formulation. Concentration of Tween 80 and chitosan were independent variables. The optimized chitosan nanoformulation of gallic acid, rutin and their combination was explored against psoriasis using in vitro methods and HaCaT cell line. The results indicated excellent entrapment of drug in the chitosan polymer and Higuchi model of drug release. The optimized encapsulated Tween 80-coated chitosan nanoparticles containing combination of gallic acid and rutin showed the reduced keratinocyte hyperproliferation, antioxidant, anti-inflammatory and antimicrobial activity even better than the chitosan nanoparticles containing gallic acid and rutin individually in the nanoparticles. Anti-psoriasis like activity of Tween 80-coated nanoformulations of gallic acid may be attributed to faster penetration of the drug by increased permeation and fusion of drug molecules. Thus, the present in vitro investigation indicates that chitosan-based nanoformulations hold promising potential in the treatment of psoriasis. The activity was increased once we combine gallic acid and rutin.
Graphic abstract








Similar content being viewed by others

References
King-man HO, Psoriasis, Med Bull 15: 10–14
Perera GK, Meglioand P, Nestle FO (2012) Psoriasis. Ann Rev Pathol Mech 7:385–422
Gudjonsson JE, Jonhston A, Sigmundsdottir H, Valdimarsson H (2004) Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol 135:1–8
Yang DQ, Jiangand YF, Zhang Y (2005) Observation on effect of pifubing xuedu pill combined with diyin tablet in treatment of psoriasis. Chin J Integr Med 25:740–742
Zangenehand FZ, Shooshtary FS (2013) Psoriasis-types, causes and medication. INTECH Open Science. https://doi.org/10.5772/54728
Mehnert W, Mader K (2001) Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–196
Suresh PK, Singh P, Saraf S (2013) Novel topical drugs carriers as a tool for treatment of psoriasis: progress and advances. Afr J Pharm Pharmacol 7:138–147
Jadhav NR, Powar T, Shinde S, Nadaf S (2014) Herbal nanoparticles: a patent review. Asian J Pharm 8:1–12
Nan W, Ding L, Shi X, Sui XB (2018) Topical use of quercetin-loaded chitosan nanoparticles against ultraviolet B radiation. Front Pharmacol 9:826
Srivastava J, Gupta N, Kushwaha A, Umrao S, Srivastava A, Singh M (2019) Highly sensitive and selective estimation of aspartame by chitosan nanoparticles–graphene nanocomposite tailored EQCM-MIP sensor. Polym Bull 76:4431–4449
Kumar DA, Singh D, Mishra J, Shrikantand N, Pandey S (2011) Development and characterization of chitosan nanoparticles loaded with amoxycillin. Int Res J Pharm 2:145–151
Gonzalez RJ, Tarloff JB (2001) Evaluation of hepatic sub cellular Fractions for Alamar blue and MTT reductase activity. Toxicol In Vitro 15:257–259
Denizot FF, Lang RR (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:5–22
Mishra K, Ojhaand H, Chaudhury NK (2012) Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chem 130:1036–1043
Bailey J, Shaw A, Fischer R, Ryan BJ, Kessler BM, McCullagh J, Wade-Martins R, Channon KM, Crabtree MJ (2017) A novel role for endothelial tetrahydrobiopterin in mitochondrial redox balance. Free Radic Biol Med 104:214–225
Rahman H, Eswaraiahand MC, Dutta AM (2015) In-vitro anti-inflammatory and anti-arthritic activity of Oryza sativa var. Joha rice (an aromatic indigenous rice of assam). Am Eurasian J Agric Environ Sci 15:115–121
Umadevi K, Krishnaveni M (2013) Antibacterial activity of pigment produced from Micrococcus luteus KF532949. IJCAS 4:149–152
Jaisinghani RN (2017) Antibacterial properties of quercetin. Microbiol Res 8:13–14
Raza ZA, Anwar F, Abid S (2019) Multi-response optimization in impregnation of chitosan nanoparticles on polyester fabric. Polym Bull 76:3039–3058
Jonassen H, Kjoniksen AL, Hiorth M (2012) Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromol 13:3747–3756
Yadav M, Sardana I, Sharma A, Sharma N, Nagpal K, Malik P (2019) Emerging pathophysiological targets of psoriasis for future therapeutic strategies. Curr Drug Targets Infect Disord. https://doi.org/10.2174/1871526519666190617162701
Zhang X, Huang Q, Liu M, Tian J, Zeng G, Li Z, Wang K, Zhang Q, Wan Q, Deng F, Wei Y (2015) Preparation of amine functionalized carbon nanotubes via a bioinspired strategy and their application in Cu2 + removal. Appl Surf Sci 15(343):19–27
Zhang X, Huang Q, Deng F, Huang H, Wan Q, Liu M, Wei Y (2017) Mussel-inspired fabrication of functional materials and their environmental applications: progress and prospects. Appl Mater Today 7:222–238
Huang Q, Liu M, Chen J, Wan Q, Tian J, Huang L, Jiang R, Wen Y, Zhang X, Wei Y (2017) Facile preparation of MoS2 based polymer composites via mussel inspired chemistry and their high efficiency for removal of organic dyes. Appl Surf Sci 419:35–44
Ahn JM, Lee JS, Um SG, Rho BS, Lee KB, Park SG, Kim HJ, Lee Y, Chi YM, Yoon YE, Jo SH (2019) Mussel adhesive protein-conjugated vitronectin (fp-151-vt) induces anti-inflammatory activity on LPS-stimulated macrophages and UVB-irradiated keratinocytes. Immunol Invest 48:242–254
Toth IY, Szekeres M, Turcu R, Sáringer S, Illes E, Nesztor D, Tombacz E (2014) Mechanism of in situ surface polymerization of gallic acid in an environmental-inspired preparation of carboxylated core–shell magnetite nanoparticles. Langmuir 30:15451–15461
Chebil L, Rhouma GB, Chekir-Ghedira L, Ghoul M (2015) Enzymatic polymerization of rutin and esculin and evaluation of the antioxidant capacity of polyrutin and polyesculin. In: Ekinci D (ed) Biotechnology. InTech, London, pp 117–133. https://doi.org/10.5772/60413
Zhang X, Wang S, Xu L, Feng L, Ji Y, Tao L, Li S, Wei Y (2012) Biocompatible polydopamine fluorescent organic nanoparticles: facile preparation and cell imaging. Nanoscale 20:5581–5584
Liu M, Zeng G, Wang K, Wan Q, Tao L, Zhang X, Wei Y (2016) Recent developments in polydopamine: an emerging soft matter for surface modification and biomedical applications. Nanoscale 38:16819–16840
Nestle FO, Daniel H, Barker J (2009) Psoriasis. N Engl J Med 361(2009):496–509
Tse WP, Che CT, Liu K, Lin ZX (2006) Evaluation of the anti-proliferative properties of selected psoriasis-treating Chinese medicines on cultured HaCaTcells. J Ethnopharmacol 108:133–141
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shandil, A., Yadav, M., Sharma, N. et al. Targeting keratinocyte hyperproliferation, inflammation, oxidative species and microbial infection by biological macromolecule-based chitosan nanoparticle-mediated gallic acid–rutin combination for the treatment of psoriasis. Polym. Bull. 77, 4713–4738 (2020). https://doi.org/10.1007/s00289-019-02984-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02984-9