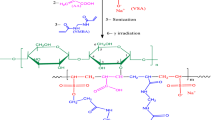
Abstract
Alginate–acrylic acid–vinylsulfonic acid/functionalized multiwalled carbon nanotubes (Alg–AA–VSA/f-MWCNTs) composite was prepared by gamma radiation as initiator the graft copolymerization of VSA and AA on Alg onto f-MWCNTs surface matching to template polymerization technique. The optimum synthesis conditions of the nanocomposite are 2.5 wt% Alg, 40 wt% comonomer, 0.5 wt% f-MWCNTs, 0.8 wt% NMBA, AA/VSA monomer composition of 70/30 wt%, and 25 kGy absorbed dose. The structure of the nanocomposite was analyzed using FTIR, TGA, SEM, and XRD. The adsorption of Co(II) radionuclide was applied by batch technique. The kinetic, isotherm, and thermodynamic data of the adsorption of Co(II) deduced that the adsorption followed the second-order model and Langmuir model and became favorable at a low temperature, respectively. Maximum capacity of Co(II) adsorption was 306.399 mg/g.











Similar content being viewed by others
References
Famá LM, Pettarin V, Goyanes SN, Bernal CR (2011) Starch/multi-walled carbon nanotubes composites with improved mechanical properties. Carbohydr Polym 83:1226–1231. https://doi.org/10.1016/j.carbpol.2010.09.027
Swain SK, Pradhan AK, Sahu HS (2013) Synthesis of gas barrier starch by dispersion of functionalized multiwalled carbon nanotubes. Carbohydr Polym 94:663–668. https://doi.org/10.1016/j.carbpol.2013.01.056
Prusty G, Das R, Swain SK (2014) Influence of functionalized single-walled carbon nanotubes on morphology, conducting and oxygen barrier properties of poly (acrylonitrile-co-starch). Compos Part B Eng 62:236–241. https://doi.org/10.1016/j.compositesb.2014.03.006
Li Y, Liu F, Xia B et al (2010) Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. J Hazard Mater 177:876–880. https://doi.org/10.1016/j.jhazmat.2009.12.114
Cheng J, Zheng P, Zhao F, Ma X (2013) The composites based on plasticized starch and carbon nanotubes. Int J Biol Macromol 59:13–19. https://doi.org/10.1016/j.ijbiomac.2013.04.010
Sand A, Yadav M, Mishra DK, Behari K (2010) Modification of alginate by grafting of N-vinyl-2-pyrrolidone and studies of physicochemical properties in terms of swelling capacity, metal-ion uptake and flocculation. Carbohydr Polym 80:1147–1154. https://doi.org/10.1016/j.carbpol.2010.01.036
Mohamed SF, Mahmoud GA, Taleb MFA (2013) Synthesis and characterization of poly(acrylic acid)-g-sodium alginate hydrogel initiated by gamma irradiation for controlled release of chlortetracycline HCl. Monatshefte fur Chemie 144:129–137. https://doi.org/10.1007/s00706-012-0776-7
Ma X, Yu J, Wang N (2008) Glycerol plasticized-starch/multiwall carbon nanotube composites for electroactive polymers. Compos Sci Technol 68:268–273. https://doi.org/10.1016/j.compscitech.2007.03.016
Castrejón-Parga KY, Camacho-Montes H, Rodríguez-González CA et al (2015) Chitosan-starch film reinforced with magnetite-decorated carbon nanotubes. J Alloys Compd 615:S505–S510. https://doi.org/10.1016/j.jallcom.2013.12.269
Xie F, Pollet E, Halley PJ, Avérous L (2013) Starch-based nano-biocomposites. Prog Polym Sci 38:1590–1628. https://doi.org/10.1016/j.progpolymsci.2013.05.002
Cao X, Chen Y, Chang PR, Huneault MA (2007) Preparation and properties of plasticized starch/multiwalled carbon nanotubes composites. J Appl Polym Sci 106:1431–1437. https://doi.org/10.1002/app.26799
Vipin AK, Ling S, Fugetsu B (2014) Sodium cobalt hexacyanoferrate encapsulated in alginate vesicle with CNT for both cesium and strontium removal. Carbohydr Polym 111:477–484. https://doi.org/10.1016/j.carbpol.2014.04.037
Hanafi A (2010) Adsorption of cesium, thallium, strontium and cobalt radionuclides using activated carbon. Asian J Chem 1:292–300. https://doi.org/10.4208/jams.100809.112309a
Caramal C, Bulgariu L, Macoveanu M (2009) Cobalt (II) removal from aqueous solutions by adsorption on modified peat moss. Chem Bull Politeh Univ 54:13–17
Fang F, Kong L, Huang J et al (2014) Removal of cobalt ions from aqueous solution by an amination graphene oxide nanocomposite. Elsevier, Amsterdam
Fang XH, Fang F, Lu CH, Zheng L (2017) Removal of Cs+, Sr2+, and Co2+Ions from the mixture of organics and suspended solids aqueous solutions by zeolites. Nucl Eng Technol 49:556–561. https://doi.org/10.1016/j.net.2016.11.008
El-Zakla T, Yakout SM, Rizk MA et al (2011) Removal of Cobalt-60 and Caesium-134 ions from contaminated solutions by sorption using activated carbon. Adsorpt Sci Technol 29:331–344. https://doi.org/10.1260/0263-6174.29.3.331
Parab H, Joshi S, Sudersanan M et al (2010) Removal and recovery of cobalt from aqueous solutions by adsorption using low cost lignocellulosic biomass-coir pith. J Environ Sci Heal Part A Toxic/Hazard Subst Environ Eng 45:603–611. https://doi.org/10.1080/10934521003595662
Prabakaran R, Arivoli S (2013) Removal of cobalt (II) from aqueous solutions by adsorption on low cost activated carbon. Int J Sci Eng Technol Res 2:271–283
Vafajoo L, Cheraghi R, Dabbagh R, McKay G (2018) Removal of cobalt (II) ions from aqueous solutions utilizing the pre-treated 2-Hypnea Valentiae algae: equilibrium, thermodynamic, and dynamic studies. Chem Eng J 331:39–47. https://doi.org/10.1016/j.cej.2017.08.019
Zhang X, Wang X, Chen Z (2017) Radioactive cobalt(II) removal from aqueous solutions using a reusable nanocomposite: Kinetic, isotherms, and mechanistic study. Int J Environ Res Public Health 14:1453. https://doi.org/10.3390/ijerph14121453
Liang S, Li G, Tian R (2016) Multi-walled carbon nanotubes functionalized with a ultrahigh fraction of carboxyl and hydroxyl groups by ultrasound-assisted oxidation. J Mater Sci 51:3513–3524. https://doi.org/10.1007/s10853-015-9671-z
Sand A, Yadav M, Behari K (2010) Preparation and characterization of modified sodium carboxymethyl cellulose via free radical graft copolymerization of vinyl sulfonic acid in aqueous media. Carbohydr Polym 81:97–103. https://doi.org/10.1016/j.carbpol.2010.02.001
Mishra MM, Sand A, Mishra DK et al (2010) Free radical graft copolymerization of N-vinyl-2-pyrrolidone onto k-carrageenan in aqueous media and applications. Carbohydr Polym 82:424–431. https://doi.org/10.1016/j.carbpol.2010.04.080
Kim MK, Sundaram KS, Iyengar GA, Lee KP (2015) A novel chitosan functional gel included with multiwall carbon nanotube and substituted polyaniline as adsorbent for efficient removal of chromium ion. Chem Eng J 267:51–64. https://doi.org/10.1016/j.cej.2014.12.091
Siyam T, Abd-Elatif ZH (1999) Gamma radiation-induced preparation of poly(dimethylaminoethyl methacrylate-acrylamide-acrylic acid) as exchanger. J Macromol Sci, Pure Appl Chem 36A:417–428. https://doi.org/10.1081/MA-100101539
Siyam T, Ayoub R (1997) Gamma radiation-induced template polymerization of acrylic acid in the presence of polyactrylamide. J Macromol Sci, Pure Appl Chem 34:1727–1735. https://doi.org/10.1080/10601329708010038
Siyam T (2001) Development of acrylamide polymers for the treatment of waste water. Des Monomers Polym 4:107–168. https://doi.org/10.1163/156855500300203377
Abdel-Aziz HM, Hanafi HA, Abozahra SF, Siyam T (2011) Preparation of poly(acrylamide-maleic acid) resin by template polymerization and its use for adsorption of co(II) and ni(II). Int J Polym Mater Polym Biomater 60:89–101. https://doi.org/10.1080/00914037.2010.504166
Borai EH, Hamed MG, El-kamash AM et al (2015) Synthesis, characterization and application of a modified acrylamide–styrene sulfonate resin and a composite for sorption of some rare earth elements. New J Chem 39:7409–7420. https://doi.org/10.1039/C5NJ01479D
Moustafa KA, Abdel-Aziz HM, Siyam T (2008) Synthesis of poly(acrylamide-acrylic acid) resin and its use for biological applications. J Radiat Res Appl Sci 1:233–244
Abdel-Aziz HM, Siyam T (2011) Radiation synthesis of poly(acrylamide-acrylic acid-dimethylaminoethyl methacrylate) resin and its use for binding of some anionic dyes. Water Air Soil Pollut 218:165–174. https://doi.org/10.1007/s11270-010-0632-5
Senna MM, Siyam T, Mahdy S (2004) Physico-chemical studies on Cu(II) complexes of acrylate chelating polymers. J Macromol Sci, Pure Appl Chem 41A:1187–1203. https://doi.org/10.1081/MA-200026568
El-Zahhar AA, Abdel-Aziz HM, Siyam T (2006) Some synthesized polymeric composite resins for the removal of Co(II) and Eu(III) from aqueous solutions. J Radioanal Nucl Chem 267:657–664. https://doi.org/10.1007/s10967-006-0099-4
Sallam KM, Abdel-Ghany IY, Abdel-Aziz HM, Siyam T (2011) Comparative study of estradiol trapped in polymer gels versus estradiol conjugated to bovine serum albumin for production of polyclonal antibody. J Radioanal Nucl Chem 289:647–652. https://doi.org/10.1007/s10967-011-1133-8
Kim SJ, Park SJ, Kim SI (2004) Properties of smart hydrogels composed of polyacrylic acid/poly(vinyl sulfonic acid) responsive to external stimuli. Smart Mater Struct 13:317–322. https://doi.org/10.1088/0964-1726/13/2/010
Hussain T, Ansari M, Ranjha NM et al (2013) Chemically cross-Linked poly(acrylic-co-vinylsulfonic) acid hydrogel for the delivery of isosorbide mononitrate. Sci World J 2013:1–9. https://doi.org/10.1155/2013/340737
Hemalatha P (2016) Studies on copoly (N-vinylpyrrolidone–acrylic acid)—as an antimicrobial. Int J Innov Res Dev 5:7–12
Yadav M, Sand A, Mishra MM et al (2012) Synthesis, characterization and applications of graft copolymer (k-carrageenan-g-vinylsulfonic acid). Int J Biol Macromol 50:826–832. https://doi.org/10.1016/j.carbpol.2009.07.026
Geethanjali R, Subhashini S (2014) Synthesis of water soluble polyvinyl alcohol-based terpolymer and evaluation of corrosion inhibition property on mild steel in hydrochloric acid. Res J Recent Sci 3:170–176
Tiwari A, Singh SP (2008) Synthesis and characterization of biopolymer-based electrical conducting graft copolymers. J Appl Polym Sci 108:1169–1177. https://doi.org/10.1002/app.27789
Singha AS, Rana AK (2011) Kinetics of graft copolymerization of acrylic acid onto cannabis indica fibre. Iran Polym J 20:913–929
Soleimani F, Sadeghi M, Shahsavari H (2012) Graft copolymerization of gelatin-g-poly (acrylic acid-co-acrylamide) and calculation of grafting parameters. Indian J Sci Technol 5:2041–2046
Lanthong P, Nuisin R, Kiatkamjornwong S (2006) Graft copolymerization, characterization, and degradation of cassava starch-g-acrylamide/itaconic acid superabsorbents. Carbohydr Polym 66:229–245. https://doi.org/10.1016/j.carbpol.2006.03.006
Abdelmonem IM, Metwally E, Siyam TE et al (2019) Radiation synthesis of starch-acrylic acid–vinyl sulfonic acid/multiwalled carbon nanotubes composite for the removal of 134Cs and 152+154Eu from aqueous solutions. J Radioanal Nucl Chem 319:1145–1157. https://doi.org/10.1007/s10967-018-6392-1
Tsubokawa N (2005) Preparation and properties of polymer-grafted carbon nanotubes and nanofibers. Polym J 37:637–655. https://doi.org/10.1295/polymj.37.637
Wang JP, Chen YZ, Ge XW, Yu HQ (2007) Gamma radiation-induced grafting of a cationic monomer onto chitosan as a flocculant. Chemosphere 66:1752–1757. https://doi.org/10.1016/j.chemosphere.2006.06.072
Blázquez G, Martín-Lara MA, Tenorio G, Calero M (2011) Batch biosorption of lead(II) from aqueous solutions by olive tree pruning waste: equilibrium, kinetics and thermodynamic study. Chem Eng J 168:170–177. https://doi.org/10.1016/j.cej.2010.12.059
Sartape AS, Mandhare AM, Jadhav VV et al (2017) Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab J Chem 10:S3229–S3238. https://doi.org/10.1016/j.arabjc.2013.12.019
Rout PR, Bhunia P, Dash RR (2015) A mechanistic approach to evaluate the effectiveness of red soil as a natural adsorbent for phosphate removal from wastewater. Desalin Water Treat 54:358–373. https://doi.org/10.1080/19443994.2014.881752
Schweitzer GK, Pesterfiel LL (2010) The Aqueous Chemistry of the Elements. New York: Oxford University Press
El-Sweify FH, Abdelmonem IM, El-Masry AM et al (2019) Adsorption behavior of Co(II) and Eu(III) on polyacrylamide/multiwalled carbon nanotube composites. Radiochemistry 61:323–330. https://doi.org/10.1134/S106636221903007X
Ibrahim AG, Saleh AS, Elsharma EM et al (2019) Chitosan’g’maleic acid for effective removal of copper and nickel ions from their solutions. Int J Biol Macromol 121:1287–1294. https://doi.org/10.1016/j.ijbiomac.2018.10.107
Saleh AS, Ibrahim AG, Abdelhai F et al (2017) Preparation of poly(chitosan-acrylamide) flocculant using gamma radiation for adsorption of Cu(II) and Ni(II) ions Alaaeldine. Radiat Phys Chem 134:33–39. https://doi.org/10.1016/j.radphyschem.2017.01.019
Ibrahim AG, Saleh AS, Elsharma EM et al (2018) Gamma radiation-induced preparation of poly(1-vinyl-2-pyrrolidone-co-sodium acrylate) for effective removal of Co(II), Ni(II), and Cu(II). Polym Bull 76:303–322. https://doi.org/10.1007/s00289-018-2379-x
Sun J, Li Y, Liu T et al (2014) Equilibrium, kinetic and thermodynamic studies of cationic red X-Grl adsorption on graphene oxide. Environ Eng Manag J 13:2551–2559. https://doi.org/10.1016/j.materresbull.2012.04.021
Suguna M, Siva Kumar N (2013) Equilibrium, kinetic and thermodynamic studies on biosorption of lead(II) and cadmium(II) from aqueous solution by polypores biomass. Indian J Chem Technol 20:57–69
Sheha RR, Metwally E (2007) Equilibrium isotherm modeling of cesium adsorption onto magnetic materials. J Hazard Mater 143:354–361. https://doi.org/10.1016/j.jhazmat.2006.09.041
Zhao J, Wang S, Zhang L et al (2018) Kinetic, isotherm, and thermodynamic studies for Ag(I) adsorption using carboxymethyl functionalized poly(glycidyl methacrylate). Polymers (Basel) 10:1090. https://doi.org/10.3390/polym10101090
Haroon H, Ashfaq T, Gardazi SMH et al (2016) Equilibrium kinetic and thermodynamic studies of Cr(VI) adsorption onto a novel adsorbent of Eucalyptus camaldulensis waste: batch and column reactors. Korean J Chem Eng 33:2898–2907. https://doi.org/10.1007/s11814-016-0160-0
Wang C, Zhao J, Wang S et al (2019) Selective capture models and mechanisms of Pb(II) from wastewater using tannic-functionalized nickel-iron oxide nanoparticles. Colloids Surf A 570:265–273. https://doi.org/10.1016/j.colsurfa.2019.03.042
Hu Q, Zhang Z (2019) Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: a theoretical analysis. J Mol Liq 277:646–648. https://doi.org/10.1016/j.molliq.2019.01.005
Dada A, Olalekan A, Olatunya A, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J Appl Chem 3:38–45
Ramachandran P, Vairamuthu R, Ponnusamy S (2011) Adsorption isotherms, kinetics, thermodynamics and desorption studies of reactive Orange16 on activated carbon derived from Ananas comosus (L.) carbon. ARPN J Eng Appl Sci 6:15–26
Stafiej A, Pyrzynska K (2007) Adsorption of heavy metal ions with carbon nanotubes. Sep Purif Technol 58:49–52. https://doi.org/10.1016/j.seppur.2007.07.008
Repo E, Warchol JK, Agustiono T, Sillanpää MET (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chem Eng J 161:73–82. https://doi.org/10.1016/j.cej.2010.04.030
Rengaraj S, Moon S (2002) Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins. Water Res 36:1783–1793
Luo W, Bai Z, Zhu Y (2017) Comparison of Co(II) adsorption by a crosslinked carboxymethyl chitosan hydrogel and resin: behaviour and mechanism. New J Chem 41:3487–3497. https://doi.org/10.1039/C6NJ03837A
Navarro A, Musaev H, Serrano K, Masud M (2014) Adsorption kinetics of cobalt(II) ions onto alginate beads from aqueous solutions. Earth Sci Clim Chang 5:1–5. https://doi.org/10.4172/2157-7617.1000223
Alqadami AA, Naushad M, Abdalla MA et al (2016) Adsorptive removal of toxic dye using Fe3O4 − TSC nanocomposite: equilibrium, kinetic, and thermodynamic studies. J Chem Eng Data 61:3806–3813. https://doi.org/10.1021/acs.jced.6b00446
Xu L, Zheng X, Cui H et al (2017) Equilibrium, kinetic, and thermodynamic studies on the adsorption of cadmium from aqueous solution by modified biomass ash. Bioinorg Chem Appl 2017:1–9. https://doi.org/10.1155/2017/3695604
Liu LE, Liu J, Li H et al (2012) Equilibrium, kinetic, and thermodynamic studies of lead (II) biosorption on sesame leaf. BioResources 7:3555–3572
Achmad A, Kassim J, Suan TK et al (2012) Equilibrium, kinetic and thermodynamic studies on the adsorption of direct dye onto a novel green adsorbent developed from Uncaria gambir extract. J Phys Sci 23:1–13
Gök C (2017) Equilibrium, kinetic and thermodynamic studies of europium adsorption by biopolymeric composite. Int J Chem Eng Appl 8:334–339. https://doi.org/10.18178/ijcea.2017.8.5.679
Huang Q, Lin X, Xiong L et al (2017) Equilibrium, kinetic and thermodynamic studies of acid soluble lignin adsorption from rice straw hydrolysate by a self-synthesized macro/mesoporous resin. RSC Adv 7:23896–23906. https://doi.org/10.1039/c7ra01058c
El-Naggar IM, Zakaria ES, Ali IM et al (2012) Kinetic modeling analysis for the removal of cesium ions from aqueous solutions using polyaniline titanotungstate. Arab J Chem 5:109–119. https://doi.org/10.1016/j.arabjc.2010.09.028
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelmonem, I.M., Metwally, E., Siyam, T.E. et al. Adsorption of 60Co from aqueous solution onto alginate–acrylic acid–vinylsulfonic acid/multiwalled carbon nanotubes composite. Polym. Bull. 77, 4631–4653 (2020). https://doi.org/10.1007/s00289-019-02978-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02978-7