Abstract
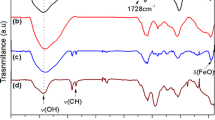
The exploration of magnetic polymer nanocomposites is of great importance owing to their unique properties and promising applications. Herein, for the first time, the ternary superparamagnetic nanocomposites of Fe3O4 nanoparticles, graphene oxide (GO) and optically active poly(amide–imide) (Fe3O4@GO/PAI) were prepared and characterized by Fourier transform infrared spectroscopy, X-ray diffraction, vibrating sample magnetometer, scanning electron microscope (SEM) and thermogravimetric analysis (TGA). The preparation was attained via a three-stage process consisting of a facile one-pot in situ growth of Fe3O4 on GO, resulted in the preparation of the magnetic Fe3O4@GO, modification of Fe3O4@GO by 3-aminopropyltriethoxy silane to introduce amino groups on its surface and subsequently its compositing by various levels of 4.0, 6.0, 8.0, and 10.0 wt% with chiral PAI through ultrasonic irradiation afforded the magnetic nanocomposites (NanoFe3O4@GO/PAI). The SEM analysis showed Fe3O4 nanoparticles with 30 nm size were successfully decorated the GO nanosheets. The TGA analysis established the expected thermal stability for Fe3O4@GO/PAI nanocomposites. Furthermore, incorporation of Fe3O4@GO in polymer matrix improved the mechanical properties substantially.








Similar content being viewed by others
References
Shen J, Hu Y, Shi M, Lu X, Qin C, Li C, Ye M (2009) Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem Mater 21:3514–3520
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053
Montes-Navajas P, Asenjo NG, Santamaria R, Menendez R, Corma A, Garcia H (2013) Surface area measurement of graphene oxide in aqueous solutions. Langmuir 29:13443–13448
Majumdar B, Sarma D, Bhattacharya T, Sarma TK (2017) Graphene oxide as metal-free catalyst in oxidative dehydrogenative C–N coupling leading to α-Ketoamides: importance of dual catalytic activity. ACS Sustain Chem Eng 5:9286–9294
Klimova K, Pumera M, Luxa J, Jankovsky O, Sedmidubsky D, Matejkova S, Sofer Z (2016) Graphene oxide sorption capacity toward elements over the whole periodic table: a comparative study. J Phys Chem C 120:24203–24212
Seabra AB, Paula AJ, de Lima R, Alves OL, Duran N (2014) Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol 27:159–168
Chung C, Kim YK, Shin D, Ryoo SR, Hong BH, Min DH (2013) Biomedical applications of graphene and graphene oxide. Acc Chem Res 46:2211–2224
Kim J, Cote LJ, Kim F, Yuan W, Shull KR, Huang J (2010) Graphene and graphene oxide sheets at interfaces. J Am Chem Soc 132:8180–8186
Basu H, Singhal RK, Pimple MV, Saha S (2018) Graphene oxide encapsulated in alginate beads for enhanced sorption of uranium from different aquatic environments. J Environ Chem Eng 6:1625–1633
Liu J, Cui L, Losic D (2013) Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater 9:9243–9257
Down MP, Rowley-Neale SJ, Smith GC, Banks CE (2018) Fabrication of graphene oxide supercapacitor devices. ACS Appl Energy Mater 1:707–714
Yadav R, Subhash A, Chemmenchery N, Kandasubramanian B (2018) Graphene and graphene oxide for fuel cell technology. Ind Eng Chem Res 57:9333–9350
Su C, Tandiana R, Balapanuru J, Tang W, Pareek K, Nai CT, Hayashi T, Loh KP (2015) Tandem catalysis of amines using porous graphene oxide. J Am Chem Soc 137:685–690
Xiao Y, Liu J, Xie K, Wang W, Fang Y (2017) Aerobic oxidation of cyclohexane catalyzed by graphene oxide: effects of surface structure and functionalization. Mol Catal 431:1–8
Huang Y, Yang HY, Ai Y (2015) DNA Single-base mismatch study using graphene oxide nanosheets-based fluorometric biosensors. Anal Chem 87:9132–9136
Chiu NF, Huang TY, Lai HC, Liu KC (2014) Graphene oxide-based SPR biosensor chip for immunoassay applications. Nanoscale Res Lett 9:445–451
Ouyang K, Zhu C, Zhao Y, Wang L, Xie S, Wang Q (2015) Adsorption mechanism of magnetically separable Fe3O4/graphene oxide hybrids. Appl Surf Sci 355:562–569
Sharafeldin M, Bishop GW, Bhakta S, El-Sawy A, Suib SL, Rusling JF (2017) Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens Bioelectron 91:359–366
Muthukrishnaraj A, Manokaran J, Vanitha M, Thiruvengadaravi KV, Baskaralingam P, Balasubramaniana N (2015) Equilibrium, kinetic and thermodynamic studies for the removal of Zn(II) and Ni(II) ions using magnetically recoverable graphene/Fe3O4 composite. Desalination Water Treat 56:2485–2501
Ali SS, Tang X, Alavi S, Faubion J (2011) Structure and physical properties of starch/poly vinyl alcohol/sodium montmorillonite nanocomposite films. J Agric Food Chem 59:12384–12395
Zhong W, Liu P, Tang Z, Wu X, Qiu J (2012) Facile approach for superparamagnetic CNT-Fe3O4/polystyrene tricomponent nanocomposite via synergetic dispersion. Ind Eng Chem Res 51:12017–12024
Huang YH, Chen MHC, Lee BH, Hsieh KH, Tu YK, Lin JJ, Chang CH (2014) Evenly distributed thin-film Ag coating on stainless plate by tricomponent Ag/Silicate/PU with antimicrobial and biocompatible properties. ACS Appl Mater Interfaces 6:20324–20333
Liu P, Zhong W, Wu X, Qiu J (2013) Facile synergetic dispersion approach for magnetic Fe3O4@graphene oxide/polystyrene tri-component nanocomposite via radical bulk polymerization. Chem Eng J 219:10–18
Zhong W, Liu P, Wang A (2012) Facile approach to magnetic attapulgite-Fe3O4/polystyrene tri-component nanocomposite. Mater Lett 85:11–13
Liu X, Yin J, Kong Y, Chen M, Feng Y, Yan K, Li X, Su B, Lei Q (2013) Electrical and mechanical property study on three-component polyimide nanocomposite films with titanium dioxide and montmorillonite. Thin Solid Films 544:352–356
Huang Y, Xiao C, Huang Q, Liu H, Hao J, Song L (2018) Magnetic field induced orderly arrangement of Fe3O4/GO composite particles for preparation of Fe3O4/GO/PVDF membrane. J Membr Sci 548:184–193
Abdah MAAM, Razali NSM, Lim PT, Kulandaivalu S, Sulaiman Y (2018) One-step potentiostatic electrodeposition of polypyrrole/graphene oxide/multi-walled carbon nanotubes ternary nanocomposite for supercapacitor. Mater Chem Phys 219:120–128
Mallakpour S, Rafiee Z (2011) New developments in polymer science and technology using combination of ionic liquids and microwave irradiation. Prog Polym Sci 36:1754–1765
Kim K, Lee J, Lee S, Yoo T, Han H (2018) Synthesis of new flexible diamine for applications in transparent poly(amide–imide) thin films with low residual stress. Prog Org Coat 117:130–140
Mallakpour SE, Hajipour AR, Habibi S (2002) Microwave-assisted synthesis of new optically active poly(ester-imide)s containing N,N-(pyromellitoyl)-bis-l-phenylalanine moieties. J Appl Polym Sci 86:2211–2216
Sun L, Yu H, Fugetsu B (2012) Graphene oxide adsorption enhanced by in situ reduction with sodium hydrosulfite to remove acridine orange from aqueous solution. J Hazard Mater 203:101–110
Van Krevelen DW, Hoftyzer PJ (1976) Properties of polymers, vol 3. Elsevier, Amsterdam
Acknowledgements
Funding was provided by Yasouj University (Grant No. Gryu-89131307).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Talebi, F., Rafiee, Z. Superparamagnetic nanocomposites: prepared by embedding Fe3O4@graphene oxide in chiral poly(amide–imide). Polym. Bull. 77, 2059–2071 (2020). https://doi.org/10.1007/s00289-019-02859-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02859-z