Abstract
Several alkali metal salts that activated 18-crown-6 were applied as initiators of β-butyrolactone anionic ring-opening polymerization in tetrahydrofuran at room temperature. Some of them, i.e., Ph2PK, Ph3HBK, (Me3Si)2NK, and t-BuOK, deprotonate the monomer which results in macromolecules with trans-crotonate starting group. Other salts, for example, MeOK, i-PrOK, PhCH2OK, and CbK (Cb denotes carbazolyl group), deprotonate monomer or open its ring in the acyl-oxygen position. After ring-opening KOH forms as intermediate and initiates further polymerization. Ph3CK and monopotassium salt of ethylenediaminetetraacetic acid also deprotonate monomer and open its ring, however, in the alkyl-oxygen position. Monopotassium salts of glycolic acid, diglycolic acid, or malonic acid initiate polymerization mainly by ring opening in the alkyl-oxygen position. It results in the formation of polymers with two reactive terminal groups. The salts used react with the monomer as strong bases, nucleophilic bases, or nucleophiles. It determines unsaturation of the polymers obtained in the wide range of 2–100 mol%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactones are important monomers, which are easily polymerized by ring-opening mechanism giving biodegradable polyesters [1, 2]. Anionic polymerization of β-lactones yields polyesters exhibiting potential utility in medicine and environmental protection [3,4,5,6,7]. Initiators, which are most frequently used for β-lactones anionic polymerization, are potassium salts of carboxylic acids. These systems involve, for example, potassium acetate, crotonate, or benzoate [8, 9]. Dibenzo-18-crown-6 (DB18C6) was applied for their activation in some cases. During the initiation and propagation, ring opening of the monomer occurs in the alkyl-oxygen position. The second class of initiators used is potassium alkoxides. Penczek et al. [10] stated that MeOK initiates polymerization of β-propiolactone (β-PL) by nucleophilic ring opening in two positions, i.e., alkyl-oxygen and acyl-oxygen in almost equal proportion. On the other hand, Dale et al. [11] reported that during the initiation of β-PL polymerization mediated with t-BuOK deprotonation of the monomer takes place. Similar effect was observed by Kircheldorf et al. [8] for β-butyrolactone (β-BL) polymerization in the presence of the same initiator. Contrary to these results, Jedliński et al. [12] stated that MeOK and t-BuOK activated with 18-crown-6 (18C6) initiates β-PL and β-BL polymerization exclusively by nucleophilic ring opening in the acyl-oxygen position. The authors proposed that the primary reaction product is unstable and eliminates KOH, which becomes the real initiator. The course of β-BL polymerization initiated with KOH activated with macrocyclic ligands was recently presented in details by us in [13]. We reported that KOH behaves in such systems as nucleophilic base, which deprotonates the monomer, as well as opens lactone ring in the acyl-oxygen position. However, exclusive deprotonation of β-BL during initiation was observed in the presence of strong bases, i.e., potassium hydride [14] and potassium naphthalenide [15] (both activated with 18C6) as well as with potassium 4-chlorothiophenolate [8]. In all β-BL polymerizations, the growing species are carboxylate anions.
The aim of present work was to reinvestigate the mechanism of β-BL polymerization initiated with potassium alkoxides activated with 18C6. MeOK, 18C6-CH2OK, i-PrOK, t-BuOK, and PhCH2OK were chosen for the study. Comparatively, several other alkali metal salts activated with macrocyclic ligands were tested as potential initiators of polymerization, namely NaH, KH, Ph3HBK, Ph4BK, Ph3CK, Ph2PK, (Me3Si)2NK and CbK (Cb denotes carbazolyl group). Monopotassium salts of glycolic acid, diglycolic acid, malonic acid, and ethylenediaminetetraacetic acid were also applied for synthesis of polyfunctional reactive poly(β-butyrolactone)s. All processes were carried out in tetrahydrofuran solution at room temperature. MALDI-TOF and NMR techniques were used for determination of chemical structure of the polymers obtained. Molar masses and dispersities of polymers were estimated by SEC chromatography.
Experimental
Materials
β-Butyrolactone (Aldrich) was heated over CaH2 for 6 h under a dry argon atmosphere and then distilled in vacuum; the fraction boiling at 47 °C/5 mmHg was collected (purity 99.6%). Distillation over metallic sodium is not recommended because the metal reacts with lactone [16]. Anhydrous tetrahydrofuran (THF) (Acros Organics) was kept over CaH2 and distilled at 66 °C. Potassium hydride (KH) was purified according to the procedure described by Brown [17]. A 35 wt% dispersion of KH in mineral oil (Aldrich) was mixed with n-pentane in a dry argon atmosphere and then decanted. It was repeated three times followed by a threefold washing with dry THF. Finally, the solvent was evaporated in vacuum. Methanol, 18-crown-6-methanol, isopropanol, and benzyl alcohol (Aldrich) were dried over molecular sieves. Glycolic acid, diglycolic acid, malonic acid, and ethylenediaminetetraacetic acid (Aldrich) were used without purification. NaH, Ph2PK, Ph3HBK, Ph4BK, (Me3Si)2NK, Ph3CH, carbazole, and t-BuOK (1.0 M solution in THF) (Aldrich) as well as coronand 18-crown-6 and cryptand C222 (Merck) were also used as received.
Initiators synthesis and polymerization
All potassium alkoxides (except t-BuOK), monopotassium salts of carboxylic acids as well as Ph3CK and CbK were synthesized in the reaction of appropriate substrate with KH activated with 18C6 in THF solution at 20 °C. All syntheses were performed in a 50 cm3 reactor equipped with a magnetic stirrer and a Teflon valve enabling substrates delivery and sampling under argon atmosphere. During the reactions gaseous hydrogen was evolved. For example, KH (0.08 g, 2.0 mmol), 18C6 (0.53 g, 2.0 mmol) and THF (15.2 cm3) was introduced into the reactor and then methanol (0.064 g, 2.0 mmol) was added by use of microsyringe. The reaction mixture was then stirred for 1 h until all H2 (44.7 cm3) was evolved. This resulted in a fine dispersion of pure anhydrous potassium methoxide in the ether medium. That system was used as the initiator when β-butyrolactone (3.44 g, 40 mmol) was introduced into the reactor. Thus, the initial concentration of the monomer was 2.0 mol/dm3, and the initial concentration of the initiator was 0.1 mol/dm3. The reaction mixture was then stirred for several days. At 99% conversion of the monomer, methyl iodide was added to it as a quenching agent. After separation of potassium iodide precipitate from the system by filtration, THF was evaporated from the solution in vacuum at 50 °C, yielding a colorless viscous polymer. However, in some cases after complete conversion of the monomer, the reaction mixture was neutralized with HCl/H2O system (0.1 mol/dm3, 50 cm3) and transferred to the separator containing chloroform (70 cm3). After shaking during 5 min, two layers were obtained, i.e., interferior polyether layer and a superior layer containing water and KCl. These layers were separated, and superior layer was removed. After three washings with distilled water, polyester was obtained by evaporating of chloroform and water in vacuum at elevated temperature. The final conversion was usually 98–99% after 4 weeks which was estimated by chromatographic method. The yields of the reactions were 96–98%. Some of investigated processes were heterogeneous (e.g., initiated with CH3OK), and others were homogeneous (e.g., in the presence of Ph3CK).
Measurements
Subsequently, 100 MHz, 13C NMR spectra were recorded in CDCl3 at 25 °C on a BruckerAvance 400 pulsed spectrometer equipped with 5-mm broadband probe and applying Waltz16 decoupling sequence. Chemical shifts were referenced to tetramethylsilane serving as an internal standard. To obtain a good spectrum of the polymer main chain exhibiting its microstructural details, about 3000 scans were satisfactory, but in order to observe the signals of the polymer chain ends, more then 10,000 scans were necessary. Molar masses and dispersities of polymers were obtained by means of size exclusion chromatography (SEC) on a Shimadzu Prominance UFLC instrument at 40 °C on a Shodex 300 mm × 8 mm OHpac column using tetrahydrofuran as a solvent. The detector was low-temperature evaporative light scattering detector (ELSD-LTII) and with polystyrenes used as calibration standards. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) spectra were recorded on a Shimadzu AXIMA Performance instrument. The mass spectrometer operated in linear mode. The laser power was optimized to obtain a good signal-to-noise ratio after averaging 250 single-shot spectra. Dithranol was used as a matrix (10.0 mg/mL) and polymer samples were dissolved in tetrahydrofuran (2.0 mg/mL) producing clear, homogenous solutions. By using a pipette, 0.5 μL of sample solution and 0.5 μL of matrix solution were applied onto a stainless-steel target plate and then air-dried at room temperature for several minutes. Data were acquired in continuum mode until acceptable averaged data were obtained and were analyzed using Shimadzu Biotech Launchpad program.
Results and discussion
Reinvestigation of initiation course of β-BL polymerization with potassium alkoxides activated with 18C6
It was observed that potassium alkoxides nonactivated with macrocyclic ligand did not initiate β-BL polymerization at room temperature. However, in the presence of ligand 18C6, which strongly complexes potassium cation, polymerization proceeded with relatively short time and high conversion of the monomer. It resulted from the high activation of growing species, which converted the contact or solvent separated ion pairs into crown separated ion pairs and free ions [3, 18].
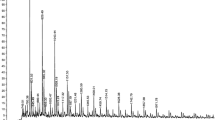
MALDI-TOF spectra of polymers obtained in the presence of potassium alkoxides activated with 18C6, i.e., MeOK, 18C6-CH2OK, EtOK, i-PrOK, and PhCH2OK, are very similar. Figure 1 shows, for example, the spectrum of poly(β-BL) prepared with use of i-PrO− (a circle denotes 18C6).
(a circle denotes 18C6).
The series of signals at m/z 844.9 to 2736.6 represents potassium adduct ions of macromolecules with hydroxyl and methoxycarbonyl end groups. The former formed at the early stage of the polymerization and the latter resulted from quenching of potassium carboxylate end growing species with MeI. For example, the peaks at m/z 1016.8, 1531.9, and 2047.6 represent ions of macromolecules A (Scheme 1) with 11, 17, and 23 β-butyrolactone units (Mcalc = 1018.1, 1534.6 and 2051.2), respectively.
The second series of peaks at m/z 741.1 to 2976.7 represents potassium adduct ions of macromolecules with trans-crotonate and methoxycarbonyl end groups. For example, the peaks at m/z 998.8, 1686.3 and 2718.2 represent potassium adduct ions of macromolecules B (Scheme 2) with 10, 18 and 30 β-butyrolactone units (Mcalc = 1000.1, 1688.8 and 2721.9), respectively.
Analysis of this polymer by 13C NMR method confirms the formation of macromolecules A and B. The spectrum reveals the signals of trans– CH3CH = CHCOO– (at 122.62 and 144.70 ppm) (Fig. 2), HOCH(CH3)CH2– (at 64.20 ppm) and –COOCH3 (at 51.66 ppm) end groups. No signal of end group derived from initiator, namely (CH3)2CHO– (at 22.21 ppm), was shown in the spectrum. Data characterizing the obtained polymers are presented in Table 1.
Chromatographic analysis (GC–MS) of the reaction mixture indicated the formation of trans-crotonic acid isopropyl ester. Formation of macromolecules A and B was reported recently by us in the polymerization of β-BL initiated with anhydrous KOH activated 18C6 [13]. It indicated that KOH really formed in the studied systems and initiated β-BL polymerization. It is also worth noting that elimination of KOH was observed earlier in other systems. For example [17], in the reaction of KH with acetone or other ketones, deprotonation occurs leading to the formation of potassium enolate. This compound adds to the carbonyl group of ketone giving the intermediate product, which decomposes by elimination of KOH. Figure 3 shows chromatogram of poly(β-BL) prepared with use of i-PrO− and quenched with MeI.
and quenched with MeI.
Scheme 3 presents new corrected version of β-butyrolactone polymerization, in which alkoxy and hydroxyl anions behave as nucleophilic bases in initiation step. On the other hand, MALDI-TOF analysis of the polymer prepared with the use of t-BuO− (5) indicated that this initiator mainly deprotonates β-BL resulting in the formation of macromolecules B. In this case, only weak signals of macromolecules A were shown in MALDI-TOF spectrum. No signal of (CH3)3CO– end group was found in 13C NMR spectrum of the polymer. It indicates that t-butoxide anions behave during the initiation step mainly as a strong base. Moreover, 13C NMR and MALDI-TOF analysis of all the polymers obtained by potassium alkoxides indicated the absence of macromolecules with –COOH end groups. It means that chain transfer reactions to the monomer, ROH or H2O, were strongly limited.
(5) indicated that this initiator mainly deprotonates β-BL resulting in the formation of macromolecules B. In this case, only weak signals of macromolecules A were shown in MALDI-TOF spectrum. No signal of (CH3)3CO– end group was found in 13C NMR spectrum of the polymer. It indicates that t-butoxide anions behave during the initiation step mainly as a strong base. Moreover, 13C NMR and MALDI-TOF analysis of all the polymers obtained by potassium alkoxides indicated the absence of macromolecules with –COOH end groups. It means that chain transfer reactions to the monomer, ROH or H2O, were strongly limited.
Determination of initiation course of β-BL polymerization with alkali metal salts of H−, B−, N−, P− and C−anions
Several other anionic systems were also applied for β-BL polymerization. It was observed that such species as NaH, NaH/15C5, NaH/18C6, KH KH/15C5, and Ph4BK/18C6 did not initiate polymerization. Others indicate various behaviors in the initiation step (Table 2). Some of them behave as strong bases, i.e., NaH/C222, KH/C222, (Me3Si)2NK/18C6, Ph2PK/18C6 or Ph3HBK/18C6. MALDI-TOF and 13C NMR spectra of polymers prepared with these initiators indicate exclusive formation of macromolecules B. Other salts, namely CbK/18C6 and Ph3CK/18C6, react as nucleophilic bases, i.e., deprotonate monomer or open monomer ring in the acyl-oxygen or alkyl-oxygen positions, respectively. Polymer prepared with CbK/18C6 contains macromolecules A and B. However, polymer obtained with Ph3CK/18C6 contains macromolecules B and also C (Scheme 4) possessing Ph3C end group. MALDI-TOF spectrum of the polymer is shown in Fig. 4.
The first series of signals at m/z 485.0 to 3062.9 represent macromolecules B as adducts with potassium ions. For example, the peaks at m/z 1255.5, 1857.4, and 2718.6 represents adducts of B with 13, 20, and 30 β-butyrolactone units (Mcalc = 1257.4, 1861.0 and 2721.9). The second series represents macromolecules C as adducts with potassium ions. For example, the signals at m/z 1155.6, 1758.6 and 2358.7 represents adducts of C containing 10, 17 and 24 β-butyrolactone units (Mcalc = 1158.0, 1760.6 and 2363.2), respectively. 13C NMR analysis of the polymer confirmed the formation of macromolecules with trans-crotonate, Ph3C (at 125.81, 127.71, 129.44, 147.43 and 59.52 ppm) and –COOCH3 end groups (Scheme 5).
Molar masses (Mn) of polymers synthesized in the presence of potassium alkoxides and other salts are in the range 1750–1950 (Mcalc = 1720). It means, that efficiency of initiators used is very high (f = 0.90–0.99). Moreover, relatively low dispersities of the polymers (Mw/Mn = 1.15–0.28) indicate that initiation occurs rather quickly.
Synthesis of poly(β-BL)s with reactive terminal groups
Monopotassium salts of several carboxylic acids were prepared and applied for initiation of β-BL polymerization in order to obtain reactive polyfunctional polyesters [19,20,21]. The results of polymers analysis are presented in Table 3.
13C NMR analysis of polymers prepared by use of monopotassium salt of malonic acid (14) or diglycolic acid (15) indicated very low content of trans-crotonate end groups in the polymers. MALDI-TOF technique confirmed the presence of two carboxyl end groups in these polymers after protonation. Figure 5 presents, for example, MALDI-TOF spectrum of polymer 14.
The spectrum reveals that the series of signals at m/z 1187 to 4542.5 represents macromolecules containing two terminal carboxyl groups. For example, peaks at m/z 1360.5, 2051.9, and 3430.6 represents macromolecules possessing 14, 22 and 38 monomer units (Mcalc = 1362.4, 2051.2, and 3428.8, respectively). They form adducts with sodium ions.
It was proposed that during the initiation the alkyl-oxygen bond cleavage takes place and propagation occurs in two directions resulting in macromolecules D after protonation (Scheme 6).
Signals of macromolecules with trans-crotonate end groups were not observed in MALDI-TOF spectrum. Strong reduction in unsaturation in this case could be explained by the presence of free carboxyl groups which possess much more active hydrogen atoms than CH2 group in the monomer. Thus, deprotonation of the latter is much more slower than that of the former.
However, application of monopotassium salt of glycolic acid activated with 18C6 (16) for initiation results in distinct increase in the polymer unsaturation. We suggest that formation of alkoxide anions in equilibrium reaction shown on Scheme 7 is mainly responsible for monomer deprotonation. In this case, the polymer growth takes place exclusively on carboxylate centers, i.e., propagation occurs in one direction.
MALDI-TOF spectrum of polymer (16) shown in Fig. 6 confirms the proposed structure.
The spectrum reveals the main series of signals at m/z 721.8 to 2269.1 which belong to macromolecules E with hydroxyl and carboxyl end groups. For example, peaks at m/z 971.6, 1578.1, and 2096.3 represent macromolecules possessing 10, 17, and 22 monomer units, respectively (Mcalc = 976.1, 1578.8, and 2095.4, respectively). They form adducts with potassium ion. The second series of signals at m/z 731.8 to 2279.1 represents macromolecules F possessing trans-crotonate and carboxyl end groups. They form adducts with potassium ion. For example, peaks at m/z 893.1, 1241.5, and 1761.0 represents macromolecules with 9, 13, and 19 monomer units (Mcalc = 900.0, 1244.4, and 1761.0, respectively).
However, the most interesting initiator used was monopotassium salt of ethylenediaminetetraacetic acid activated with 18C6 (17) due to the presence in the molecule one active carboxylate group and two nitrogen atoms with free electron pairs. It results in higher increase in unsaturation of the polymer obtained (13C NMR). We propose that deprotonation of the monomer by ethylenediamine fragment is responsible for this effect (Scheme 8). Similar mechanism was proposed earlier by Kircheldorf et al. [8] for β-BL polymerization initiated with triethylamine. Analysis of the polymer by MALDI-TOF spectrometry confirmed the proposed polymer structure. Spectrum is presented in Fig. 7.
The main series of signals at m/z 814.0 to 3827.5 which belong to macromolecules possessing trans-crotonate and carboxyl end groups. They form adducts with potassium ion. For example, peaks at m/z 1330.8, 1935.4, and 2883.4 represent macromolecules with 14, 19, and 39 monomer units (Mcalc = 1330.5, 1933.2, and 2880.2, respectively). The second series of signals at m/z 798.8 to 3811.5 belong to the same macromolecules forming adducts with sodium ion. For example, peaks at m/z 1572.1, 2346.9, and 2951.7 represents macromolecules with 17, 26, and 33 monomer units (Mcalc = 1573.9, 2347.8, and 2950.5, respectively). The third series of weak signals at m/z 934.0 to 3430.9 belong to macromolecules possessing end group derived from initiator and carboxyl end group. They form adducts with potassium ion. For example, peak at m/z 2136.3 represents macromolecules with 21 monomer units (Mcalc = 2139.4), whereas peak at m/z 2120.3 represents macromolecules with 21 monomer units which form adducts with sodium ion (Mcalc = 2123.3).
In summary, the kind of initiator used strongly influences unsaturation of polymers obtained in anionic polymerization of β-butyrolactone. It results in polymers containing trans-crotonate end groups in wide range of 2–100 mol%. Basicity/nucleophilicity ratio of initiators is responsible for this phenomenon (Scheme 9).
Conclusions
In the present work, the course of initiation step in anionic ring-opening polymerization of β-butyrolactone was studied. Several sodium or potassium salts of different anions were applied as initiators. The most important observations resulting from this work were presented below.
-
MeOK, EtOK, i-PrOK, t-BuOK, PhCH2OK, NaH, NaH/15C5, NaH/18C6, KH, KH/15C5, and Ph4BK/18C6 systems do not initiate polymerization.
-
NaH/C222, KH/C222, (Me3Si)2NK/18C6, Ph2PK/18C6, Ph3HBK/18C6, and t-BuOK/18C6 systems deprotonate monomer resulting in macromolecules with trans-crotonate end group (these initiators behave as strong bases).
-
Monopotassium salts of glycolic acid, diglycolic acid, and malonic acid activated with 18C6 open monomer ring mainly in the alkyl-oxygen position resulting in macromolecules with OH or COOH end groups. The applied initiators behave as nucleophiles.
-
MeOK, 18C6-CH2OK, EtOK, i-PrOK, PhCH2OK, and CbK activated with 18C6 deprotonate monomer or open its ring in the acyl-oxygen position resulting in elimination of KOH/18C6, which becomes real initiator of the polymerization; in these systems, macromolecules with OH or trans-crotonate end groups are created. The applied initiators behave as nucleophilic bases.
-
Ph3CK and monopotassium salt of ethylenediaminetetraacetic acid activated with 18C6 deprotonate monomer or open its ring in the alkyl-oxygen position resulting in macromolecules with Ph−3C or trans-crotonate end groups. The applied initiators behave also as nucleophilic bases.
References
Monsalve M, Contreras JM, Laredo E, López-Carrasquero F (2010) Ring-opening copolymerization of (R, S)-β-butyrolactone and ε-caprolactone using sodium hydride as initiator. Express Poly Lett 4:431–441. https://doi.org/10.3144/expresspolymlett.2010.54
Labet M, Thielemans W (2009) Synthesis of polycaprolactone: a review. Chem Soc Rev 38:3484–3504. https://doi.org/10.1039/b820162p
Penczek S, Cypryk M, Duda A, Kubisa P, Słomkowski S (2007) Living ring-opening polymerizations of heterocyclic monomers. Prog Polym Sci 32:247–282. https://doi.org/10.1016/j.progpolymsci.2007.01.002
Jedliński Z (1998) Regioselective ring-opening anionic polymerization of β-lactones. Macromol Symp 132:377–383. https://doi.org/10.1002/masy.19981320135
Juzwa M, Jedliński Z (2006) Novel synthesis of poly(3-hydroxybutyrate). Macromolecules 39:4627–4630. https://doi.org/10.1021/ma0602369
Jedliński Z, Kurcok P, Lenz RW (1998) First facile synthesis of biomimetic poly-(R)-3-hydroxybutyrate via regioselective anionic polymerization of (S)-β-butyrolactone. Macromolecules 31:6718–6720. https://doi.org/10.1021/ma980663p
Rowley JM, Lobkovsky EB, Coates G (2007) Catalytic double carbonylation of epoxides to succinic anhydrides: catalyst discovery, reaction scope, and mechanism. J Am Chem Soc 129:4948–4960. https://doi.org/10.1021/ja066901a
Kricheldorf HR, Scharnagl N (1989) Polyactones. 17. Anionic polymerization of β-D, L-butyrolactone. J Macromol Sci A26:951–968. https://doi.org/10.1080/00222338908052023
Duda A (1992) Anionic polymerization of 4-methyl-2-oxetanone (β-butyrolactone). J Polym Sci, Part A: Polym Chem 30:21–29. https://doi.org/10.1002/pola.1992.080300103
Hofman A, Słomkowski S, Penczek S (1984) Structure of active centers and mechanism of the anionic polymerization of lactones. Macromol Chem Phys 185:91–101. https://doi.org/10.1002/macp.1984.021850110
Dale J, Schwartz J-E (1986) Macrocyclic oligolactones by oligomerization of simple lactones. Acta Chem Scand B40:559–565. https://doi.org/10.3891/acta.chem.scand.40b-0559
Jedliński Z, Kowalczuk M, Kurcok P (1991) What is the real mechanism of anionic polymerization of β-lactones by potassium alkoxides? A critical Approach. Macromolecules 24:1218–1219. https://doi.org/10.1021/ma00005a042
Grobelny Z, Matlengiewicz M, Skrzeczyna K, Swinarew A, Golba S, Jurek-Suliga J, Michalak M, Swinarew B (2015) Ring-opening polymerization of lactones initiated with metal hydroxide-activated macrocyclic ligands: determination of mechanism and structure of polymers. Int J Polym Anal Charact 20:457–468. https://doi.org/10.1080/1023666X.2015.1036219
Kurcok P, Matuszowicz A, Jedliński Z (1995) Anionic polymerization of β-lactones initiated with potassium hydride. A convenient route to polyester macromonomers. Macromol Rapid Commun 16:201–206. https://doi.org/10.1002/marc.1995.030160308
Jedliński Z, Kowalczuk M, Główkowski W, Grobelny J, Szwarc M (1991) Novel polymerization of β-butyrolactone initiated by potassium naphthalenide in the presence of a crown ether or a cryptand. Macromolecules 24:349–352. https://doi.org/10.1021/ma00002a002
Grobelny Z, Stolarzewicz A, Morejko B, Pisarski W, Maercker A, Skibiński A, Krompiec S, Rzepa J (2006) C–O and not C–C bond cleavage starts the polymerization of β-butyrolactone with potassium anions of alkalide. Macromolecules 39:6832–6837. https://doi.org/10.1021/ma052511h
Brown CA (1974) Saline hydrides and superbases in organic reactions. VII. Potassium hydride, highly active new hydride reagent. Reactivity, applications, and techniques in organic and organometallic reactions. J Org Chem 39:3913–3928. https://doi.org/10.1021/jo00940a025
Szwarc M (1974) Ions and ion pairs in organic reactions. Wiley, New York
Alfei S, Catena S (2018) Synthesis and characterization of fourth generation polyester-based dendrimers with cationic amino acids-modified crown as promising water soluble biomedical devices. Polym Adv Technol. https://doi.org/10.1002/pat.4396
Das T, Sengupta S, Bandyopadhyay A (2018) Part I—synthesis of hyperbranched polymers: step-growth methods. In: Hyperbranched polymers for biomedical applications. springer series on polymer and composite materials, Springer, Singapore, pp 15–63
Pirozhnikov PB, Korolev IV, Kuzina NG, Mashlyakovskii LN (2013) Hyperbranched polymers and their use in the technology of paint-and-varnish materials and coatings (A review). Russ J Appl Chem 86(10):1549–1562. https://doi.org/10.1134/S1070427213100133
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Grobelny, Z., Golba, S. & Jurek-Suliga, J. Ring-opening polymerization of β-butyrolactone in the presence of alkali metal salts: investigation of initiation course and determination of polymers structure by MALDI-TOF mass spectrometry. Polym. Bull. 76, 4951–4966 (2019). https://doi.org/10.1007/s00289-018-2640-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2640-3