Abstract
The homopolymer (PANI) and its homologue PANI–PEO2000 copolymer have been prepared under effect of Maghnite-H+ (Algerian MMT) in different weight percentages (wt%) by cationic polymerization method. The formation of the hydrogen bonding between PANI and PEO2000 and structure of PANI are predicted by the FT-IR and 1HNMR spectra. Glass transition temperature of pure PANI and pure PEO2000 are, respectively, Tg = 74 °C and Tg = 65 °C, but DSC analysis for the copolymer (PANI–PEO2000) showed only one (Tg = 16.79 °C) implying compatibility of PANI and PEO2000. Here we can suggest that under suitable condition (the amount of catalyst, molecular weight and functionality of the reactive stabilizer (PEO2000) and molar ratio of (PANI/PEO2000), our catalyst can improve the morphology, structure and chemical properties of PANI and its homologue PANI–PEO2000.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clay minerals especially the members of smectite group are the most suitable candidates for synthesis of polymer nanocomposites [1], because these possess a unique structure and reactivity together with high strength [2]. In general, the structures of polymer/clay nanocomposites are classified according to the level of intercalation and exfoliation of polymer chains into the clay galleries [3]. Various parameters including clay nature, organic modifier and polymer matrix and preparation method are affective on the intercalation and exfoliation level [4]. Therefore, depending on the nature and properties of clay and polymer as well as preparation methodology of nanocomposites, different composite microstructures can be obtained [5]. For commercial applications, PANI homopolymer and its homologue (PANI–PEO) are the best promising materials in conducting polymers, because of environmental stability [6], easy processing [7] and economical efficiency [8]. PANI has been used for electrode of light emitting diode [9], Li-ion rechargeable battery [10] and corrosion protection [2]. Polymer electrolytes have received considerable attention due to their potential uses in solid-state electrochemical devices and particularly in lithium batteries [11]. Nowadays the efforts are on the addition of plasticizers to the polymer electrolytes for improving their ionic conductivity [12]. In some cases, a large amount of plasticizers is required to dissolve the salts, and the materials are referred to as polymer gel electrolytes [13]. Among organic–inorganic nanocomposites, PANI–MMT and PANI–PEO–MMT nanocomposites are the most prevalent and interesting due to the special properties as well as wide uses of polyaniline [14], the nature, abundance, low cost of MMT and attractive features such as a large surface area and ion-exchange properties [15]. The clay, which has been used as catalyst, is supplied by a local company known as ENOF Maghnia (Western of Algeria) [16]. The greatest proton saturation of the < 2 mm fractions of clay was obtained by first saturating with Na+ ions using 1 M NaCl solution, then the protonated forms of montmorillonite (Mag-H+) were prepared by shaking the clay in a solution of sulfuric acid 0.25 M until saturation was achieved (normally after 2 days at room temperature) [17]. The cation-exchanged clay was then recovered by filtration and again suspended in deionized water. This process was repeated until no sulfate ions were indicated and present in the filtrate using BaCl2. The Mag-H+ was then isolated by filtration, dried at 105 °C and then finely ground. The cation exchange capacity (CEC) and surface area of the clay were found to be 84 mEq (100 g−1) of dried clay and 786 m2 g−1, respectively [18].
However, traditional polymerization techniques show some practical disadvantages (e.g., requirement for extremely pure reagents, low functional group tolerance, limited combination with other monomers or polymer segments). It has long been known that molecules undergo excitation with electromagnetic radiation. This effect is utilized in household microwave ovens to heat up food. However, chemists have only been using microwaves as a reaction methodology for a few years. Some of the first examples gave amazing results, which led to a flood of interest in microwave-accelerated synthesis. Microwave heating has been found to be particularly advantageous for reactions under “dry” media. Enormous accelerations in reaction time can be obtained if overheating is carried out in closed containers under high pressure; a reaction which takes several hours under conventional conditions can be completed in a few minutes; in addition in the absence of solvent on a solid support with or without a catalyst, it offers a certain number of advantages: the solvents are often expensive, toxic and difficult in the case of high-boiling aprotic solvents [19]. Moreover, the absence of solvent reduces the risk of explosions when reaction takes place in a microwave oven [20]. Reactions under “dry” conditions were originally developed in the late eighties [21]. Synthesis without solvents under microwave irradiation offers several advantages [22]. The absence of solvent reduces the risk of explosions when the reaction takes place in a closed vessel in an oven [23]. Moreover, aprotic dipolar solvents with high boiling points are expensive and difficult to remove from the reaction mixtures [24]. During microwave induction of reactions under dry conditions, the reactants are adsorbed on the surface of alumina, silica gel and clay [25]. Consequently, such supported reagents efficiently induce reactions under safe and simple conditions with domestic microwave ovens instead of specialized expensive commercial microwave systems [26]. Many researchers have studied cationic polymerization of polyaniline using “H2O”/KPS initiator system and CH2Cl2 solvent [27, 28]. Frequently, these initiators require the use of very high or very low temperature and high pressures during the polymerization reaction. The separation of the initiators from the polymer is not always possible. Therefore, the presence of toxic initiators presents problems in the manufacture of polymers used especially in medical and veterinary procedures. The purpose of this paper is to study the polymerization of aniline and its homologue block copolymer PANI–PEO catalyzed by Maghnite-H+ as proton exchanged montmorillonite clay under microwave irradiation. This new non-toxic cationic catalyst has exhibited higher efficiency via the polymerization of vinylic and heterocyclic monomers [29, 30]. This catalyst can be easily separated from the polymer product and regenerated by heating at a temperature above 100 °C [9]. The effects of different synthesis parameters, such as the amount of Maghnite-H+, the molar ratio (aniline/PEO) and reaction time, on the yield of polymerization are discussed together with the mechanism of polymerization.
Experimental
Microwave apparatus
Microwave irradiation was performed in a single-mode focused CEM reactor (Model Discover, CEM Co., Matthew, NC) operating at 2.45 GHz with ability to control output power. Temperature in the system was measured by a fiber optic temperature sensor preventing interaction with MWs and influence on the temperature reading. The heat capacity Cp of the solution was approximated as the heat capacity of water. All experiments were done under the same conditions by keeping constant irradiation power, temperature and the initial reaction mixture volume (12 mL). With the experimental design that was used, the temperature was maintained at 160 °C in all experiments.
Materials and methods
Polyaniline (PANI) was prepared in our laboratory (laboratory of polymers chemistry, Oran University, Algeria) by standard chemical intercaled method [31]. Polyethylene oxide (relative molecular mass of 2 × 103) was obtained from Sigma Aldrich. MMT clay was obtained from ENOF Maghnia (Algeria). The MMT-H+ (Mag-H+) was prepared as described by Belbachir et al. [32], and water (pH < 7) was used to synthesize emeraldine salt clay (PANI/Mag-H+) by cationic polymerization. Some of the emeraldine base (PANI-EB), non-conducting form of polyaniline, was prepared by de-protonating PANI-ES in NaOH solution (0.5 M). A doping EB was carried out in aqueous medium of hydrochloric acid (1 M). The emeraldine base (50 mg) is doped and dissolved in (100 ml) of (2 M) HCl. For 2 h and the same time (PEO), 10 mg dissolving matrix (PEO) with (PANI) thin films of conducting polymer alloys PANI–PEO2000 is prepared by mixed different volume ratio of PANI with PEO2000 [33].
Measurements
1H nuclear magnetic resonance (NMR) measurements were carried out on a 300 MHz Bruker NMR spectrometer equipped with a probe BB05 mm, in CDCl3. Tetramethylsilane (TMS) was used as the internal standard in these cases. Fourier transform infrared spectroscopy (FT-IR) spectra were obtained between 900 and 4000 cm−1 on an ATI Matson FT-IR No 9501165. Ten scans were averaged at a resolution of 4 cm−1 for the solid tested samples of modified and unmodified montmorillonite prepared as KBr pellets (ca. 3% by mass in KBr).Viscosity measurements were carried out with an Ubbelohde capillary viscosimeter (viscologic TI1, version 3–1 Semantic). Intrinsic viscosity [η] was measured at 30 °C in benzene. GPC measurements of the samples were carried out using a WISP 712, Waters Associates chromatograph, THF was used as solvent and the instrument was calibrated to a first approximation with polystyrene of known molecular weights. The flow rate of tetrahydrofuran was 10 ml/min. Intrinsic viscosity measurements were performed on SEMATECH Viscologic TI 1 apparatus at 25 °C using THF as solvent. The purification of polymers was carried out by dissolving the product in chloroform (CHCl3) and filtering to eliminate the Maghnite-H+. Then, chloroform was removed by evaporation.
Preparation of catalyst (Maghnite-Na+)
The montmorillonite used in this work came from a quarry located in Maghnia (North West of Algeria) and was supplied by company “ENOF” (an Algerian manufacture specialized in the production of nonferric products and useful substances). The different chemical elements of the native montmorillonite were transformed into oxides and analyzed by FT-IR and X-ray fluorescence (experiment carried out at ENOF). These results confirm that the maghnite used consists essentially of montmorillonite (MMT) and has been prepared in laboratory chemistry of polymers in ORAN University (LCPO), and this clay is purified by separation of the argillaceous phase and the coarse phases. Rough clay is put in suspension in distilled water. In the suspension, the solid/liquid report/ratio is approximately 1/10. The suspension is then filtered on a sieve 0.02 mm in diameter of pores to eliminate the coarse matter and stones. It then versed in test tubes and is left at rest during 2 h. The separation of the argillaceous phase of the coarse fraction which remains at the bottom is made by siphonage. The recovered suspension is then centrifuged with 4500 tr/min during 20 min. Recovered clay is treated with a solution 1 M of sodium hexametaphosphate (NaPO3)6 (clay 20 g in 100 ml), by maintaining agitation, during 3 h. The suspension is versed then in the test tubes of separation and Na-montmorillonite is to separate while exploiting its falling speed, MMT crosses with 20 °C, a distance of 10 cm each 8 h. Therefore, Na-montmorillonite is recovered by siphonage at a distance of 20 cm after 16 h of decantation. One adds water distilled to the test tubes. After each siphonage, one agitates during 15 min and one lets the suspension be elutriated before proceeding to new a siphonage. MMT is then recovered by centrifugation with 4500 tr/min during 1 h. With the end, it is washed with distilled water (on several occasions), filtered using one sintered of porosity 3 (maximum diameter of pores from 16 to 40 μm), dried in the drying oven with 105 °C, crushed using a mortar and kept in a desiccators [33, 34].
Preparation of catalyst (Maghnite-H+)
The Maghnite-H+ was prepared according to the process similar to that described by Belbachir et al. [29, 35]. 30 g of raw maghnite was crushed for 20 min using a prolabo ceramic balls grinder. The maghnite was placed in an Erlenmeyer flask together with 100 ml of distilled water and then stirred using a magnetic stirrer for 2 h at room temperature, a solution of sulfuric acid 0.25 M was added to the mixture and stirred over 2 days at room temperature until saturation was achieved, the mineral was then washed with distilled water up to pH 7. After filtration, the activated maghnite is dried in the stove for 24 h at 105 °C.
Synthesis of PANI composite
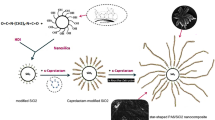
A solution of 0.25 M of sulfuric acid (H2SO4) was prepared and (5 wt%) of Maghnite-Na+ (Algerian MMT) was then added. The mixture of (Maghnite-Na+ and H2SO4) was put into a flask with 100 mL and stirred to allow proper mixing. To this end, adequate amount of aniline (ranging from 0.05 mol) was added to a solution of maghnite and sulfuric acid (0.25 M in 25 mL of distilled water) under vigorous stirring. The reaction mixture was then submitted to microwave irradiation at 160 °C and for 4 min. The mixture was cooled (5–10 min at room temperature), filtered and washed extensively with distilled water and methanol to remove maghnite and any unreacted aniline until the washing solution became neutral and air-dried.
Synthesis of copolymer PANI–PEO
We aim to prepare PANI–PEO, with different PEO weights (400, 2000 and 30.000 g/mol) starting from aniline in an acid medium. To this end, adequate amount of aniline (ranging from 0.05 mol) is added to a solution of PEO (0.05 g in 25 mL of distilled water) under vigorous stirring. Then, 5 wt% of Maghnite-H+ as an initiator was added to the above solution. The mixture was stirred for 15 min. Then it is treated in a microwave oven at the power of 950 W. Temperature and viscosity of the reactive mixture increase fast. The gelation point is reached after 4 min at 160 °C and a recognizable odor (Scheme 1).
Results and discussion
Spectroscopy characterization
Figure 1 shows the FT-IR spectra of pure polyaniline (PANI).The formation of polyaniline is confirmed by noticing the predominant peaks at the wave numbers of 1501 cm−1 corresponding to C=C stretching of quininering, 1557 cm−1 for C=C stretching of benzenoid ring, 1293 cm−1 for C–N stretching, 755 cm−1 and 838 cm−1 for C–H vibration of para coupling benzenoid and benzene rings, C–H bending at 694–593 cm−1 corresponds to aromatic ring and 507 cm−1 is stretching at out of the plane. In pure PEO2000 spectrum, a large broad band appears centered at 3442 cm−1 which is due to the hydratation of PEO2000 which confirms that PEO2000 is highly hydrophilic and gets hydrated. Thus, pure PEO2000 shows a large broad band of CH2 stretching between 2950 and 2840 cm−1. It is also been observed that CH2 vibration of all modes appear in PEO2000 at 1467 cm−1 which corresponds to asymmetric CH2 bending and 1344 cm−1 which corresponds to symmetric CH2 wagging [36].
The infrared spectra for PANI–PEO2000 copolymer is also shown in Fig. 2. There are significant changes in both the intensities and the frequencies in the product (PANI–PEO2000). They are more pronounced between 690 and 1574 cm−1. The bonds also show doublet and triplets. This may indicate that there is significant interaction between the oxygen of the ether group of polyethylene oxide and the nitrogen in the aniline of polyaniline [37].
The 1HNMR spectral of the PANI homopolymer polymer exhibits strongest sharp peaks centered at 7 ppm and 7.8 ppm due to protons from phenylene and disubstituted phenylene units, the weak peak at 4.81 ppm and medium broad peak at 6.22 ppm due to (–NH– and –NH2) end group, respectively, another broad peaks centered at 1.78 and 8 ppm may be due to the water protons bonded by (–NH– and –NH2) groups and (H–N+), respectively, as show in Fig. 3 [33].
An investigation was devoted to the analysis of the polyethylene oxide (PEO2000) by 1H NMR spectroscopy at 300 MHz (Fig. 4). According to the work published by Yahiaoui et al. [38], 1H NMR spectroscopy at 300 MHz (Solvent CDCl3) (Fig. 4) showed different peaks: (a) the methylene groups (CH2–) at 2.6 ppm and (b) the methylene (CH2O–) at 3.7 ppm.
In the 1HNMR spectra of copolymer (PANI–PEO2000), a wide signal in the region of 6.8–8 ppm was assigned to benzenoid hydrogen of polyaniline [39]. Three peaks in these region were assigned to para position of polyaniline linked for the polymerization. Signals at 3.25–3.75 ppm indicate peak of CH2O– and CH2CH2O– hydrogen of polyethylene oxide reported [40]. Peak at 1.5–2 ppm is due to CH2 hydrogen, respectively. The shift of the CH2 peak to highly shielded region is due to polymerization. Integral ratio of the NH proton signal of polyaniline in PANI–PEO2000 appeared at 4.25 ppm, to note that the chemical displacement of this peek was changed from polyaniline (4.81 ppm) to PANI–PEO2000 copolymer (4.25 ppm) which shows the copolymerization of PANI and PEO2000 (Fig. 5) [41].
Optical properties
Conductive polymers (PANI and PANI–PEO2000) have a conjugated system of double bonds in a backbone polymer. These polymers show some conventional transitions in the UV region, such as n → π* and π → π*. The π → π* transitions of conjugated double bonds are close to the visible region, associated with polaron and bipolaron states as well as solution conductive polymers [42]. The UV–visible spectral peak in the 250–300 nm region is due to the aniline groups and π → π* is a conjugated couple system of the benzoic states in the 350–400 nm regions (Fig. 6).
Thermophysical properties
Thermal stability of polyaniline/maghnite and polyaniline/PEO/maghnite composites of different composition has been analyzed and compared with that of pure PANI.
-
(A) Thermal analysis of Maghnite-H+ The thermal characterization of the composites includes thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC).
Weight losses (%) versus temperature (°C) curves for pure Maghnite-H+ are shown in Fig. 7. TGA of pure Maghnite-H+ shows two stages of weight loss. The first weight loss in maghnite below 100 °C is a result of the release of free water. The second weight loss around 600 °C is associated with the dehydroxylation of silicate structure [43]. The total weight loss is only 13.94% up to 800 °C. As can be expected, Maghnite-H+ shows a high thermal stability (Fig. 8).
-
(B) Thermal analysis of PANI The TGA thermogram of the polyaniline (PANI) at a heating rate of 10 °C/min in nitrogen is shown in Fig. 9. It can be found that at the temperature range of 187–600 °C the weight loss amounted to 61, 17%, which can be reasonably attributed to the weight loss of the polymerized polyaniline (PANI) and to thermal decomposition of the polyaniline chains [44]. Polyaniline is known to be a hygroscopic polymer [45]. Some authors assigned the endothermic effect registered by DSC in the range from ambient temperature up to approximately 120 °C to the evaporation of water.
Figure 10 is the DSC thermogram of the PANI powder in the first run and second run. In the first run, there were two endothermic peaks at 55.99 and 103.46 °C and the PANI had discernible moisture content [46]. Therefore, these endothermic peaks were most likely due to the evaporation of water. This was in agreement with the TGA results [47]. In the second run, there were almost no significant endothermic or exothermic peaks, as shown in Fig. 10, because no apparent moisture existed in the sample but Tg appears at 74.06 °C [48].
-
(C) Thermophysical properties of PANI-PEO2000were studied by TGA, TDA and DSC The weight loss (%) was recorded as function of temperature for both PANI and PEO2000 using TGA (SDT Q600 V20.9 Build 20). The samples weighing about 2.0 mg were scanned in the temperature range of 0–800 °C under nitrogen atmosphere at a heating rate of 10 °C/min. The curves of weight loss versus temperature showing the behavior of PANI–PEO2000 sample are presented in Fig. 11. The first significant weight loss occurs already at temperature between 50 and 100 °C. It is known that PANI–PEO2000 is hygroscopic and during the heating to 100 °C the residual water evaporates [49]. Then the main mass loss, which corresponds to polymer degradation, starts at about 200 °C and 500 °C [50].
Thermal behavior of pure PANI and PANI–PEO2000 was also verified by differential scanning calorimetry in which the first and second scans are shown in (Fig. 12). The first scan gives very little information on the thermal behavior of the homopolymer and copolymer. First we notice the presence of an endothermic effect (68.39 °C and 190.09 °C), the first is associated to the evaporation of water absorbed by the copolymer and the second for melting POE2000 block [51]. These second scanning will foresee it no effect on this temperature range, because the copolymer was heated to 280 °C, which facilitates the onset of glass transition temperature (Tg = 16.79 °C) which is in agreement with the literature [52] (Table 1).
Results of GPC
Gel permeation chromatography was performed with a Spectra-Physics chromatograph, equipped with four columns connected in series and packed with Ultrastyragel 103, 104, 105, 106A°, tetrahydrofuran (THF) was used as solvent and the instrument was calibrated to a first approximation with polystyrene of known molecular weights. The GPC curves for PANI and PANI–PEO indicate a bimodal distribution. The molecular weight distribution averages for the polymer and its homologue copolymer are presented in Tables 2, 3 and Figs. 13, 14. This bimodal distribution has been reported previously for PANI in (NMP) [53] and of PANI–PEO2000 in (DMSO) [54]. The molecular weight of the polymer shows the growth of PANI on PAN–maghnite. The gel permeation chromatograph (GPC) measurements of the product are in a good agreement with the PANI and poly(PANI–PEO) structure. The macromolecular weight distribution of the obtained polymer and copolymer is narrow, and this suggests that chain transfer does not occur confirming the formation of the polymer PANI and the block copolymer PANI–PEO (Table 4).
Conductivity measurement
The electrical conductivity was measured by the four-point method. Four points aligned and spaced in the same distance are applied by simple pressure on the sample. A current I is injected through the outer tips with a current source, thus creating a potential variation. Voltage U can be measured between two points connected to internal voltmeter [18, 19]. The measured value of the transverse strength of the PANI and its homologue PANI–PEO2000 are converted to volume resistivity, using Eq. (1) [55] and then the electrical conductivity is calculated from Eq. (2) [56]. In applying these equations, the value of (σ) is presented in the following Table 5:
Solubility study
Solubility of PANI and PANI–PEO2000 composites was determined in a number of organic solvents (see Table 5 and Fig. 15). The composites in powder form were added to 100 mL of each solvent including: DMF, acetonitrile, toluene, dichloromethane, THF and chloroform and stirred for 1 h before filtering. The dry weight of the filter paper was used to calculate the solubility of the composites. It was found that PANI and its homologue PANI–PEO2000 composites are relatively soluble in all above-mentioned solvents [57, 58]. The best solvents for PANI and its homologue are determined to be DMF and toluene. By UV–vis spectra, on taking the soluble PANI and PANI–PEO2000 in various solvents, we can calculate the band of energy by this equation.
The solubility parameter (δ) is usually expressed in (cal/cm3)1/2 or preferably (j/cm3)1/2 units for many compound and is defined from Hildebrand-Scotchard solution theory as:
Viscosity measurements
Viscosity measurements were carried out with an Ubbelohde capillary viscosimeter (viscologic TI1, version 3–1 Semantec). Intrinsic viscosity [η] was measured at 30 °C in benzene. Viscosity-average molecular weight Mv was calculated according to the equation: [η] (mg l−1) = K [M]a. Intrinsic viscosity and physical properties of PANI and PANI–PEO2000 are shown in Tables 6 and 7.
Kinetics studies
After having good knowledge of the physical and chemical properties of our product, we made a fairly detailed kinetics study of which we have followed the influence of the amount of catalyst, the amount of the oxidant, temperature, volume water, the time and the monomer ratio in the reaction yield, the intrinsic viscosity of the product and their average molecular weights.
Effect of the amount of Mag-H+ on the yield
Copolymerization of polyaniline by poly(ethylene oxide) induced by the Mag-H+ was carried without solvent under the effect of the microwave irradiation. Figure 9 shows the effect of the amount of catalyst on the yield of this copolymerization. As can be seen in this Fig. 16, the copolymerization rate increased with the amount of Mag-H+ and reaches a maximum at 160 °C with (5 wt% ) of catalyst, above this temperature and percentage of catalyst, the yield decreases. These results clearly indicate that the optimum conditions of these reactions are 160 °C and 5% by molecular weight of the catalyst. The increase in yield with temperature and molecular weight of catalyst is mainly due to the number of active sites in the catalyst responsible for initiating the reaction. Similar results are obtained by Ferrahi et al. [56, 59].
Effect of the amount of Mag-H+ on the molecular weight
Several reactions have summers made to follow the influence of the amount of catalyst on the molecular weight of copolymer PANI–PEO. We have used (2%, 5% and 10%) by weight as the amount of maghnite and varying time after keeping the other parameters (the amount of monomers and the temperature). The percentage by weight of Maghnite-H+/monomer is 5%. It is observed that the average molecular weight increases with time and reaches a maximum at 4 min of reaction (Fig. 10). There is also increase in molecular weight, between 2.5 and 3.5 min and decrease from 4 min. We deduce that at sufficiently long periods, degradation reactions are becoming more frequent and cause the formation of oligomers of low molecular weights. This explains the decrease in molecular weight to longer reaction times [60].
Effect of molar ratio aniline/PEO on the yield
As all the reactions were carried out in solution, it was interested to study the effect of molar ratio on the copolymerization of aniline/PEO. Under the experimental conditions (160 °C, 4 min), we used a series of aniline/PEO and maintaining the amount of the Mag-H+ (2 wt%, 5 wt% and 10 wt%), the time (4 min). All reactions were held in a temperature (160 °C) under microwave irradiation. Figure 11 summarizes the influence of the molar ratio on aniline/PEO upon the reaction yield. We notice a significant change in the yield with increasing the amount ratio, in particular with increasing the amount of PEO used in this reaction processing. Figure 11 shows that the yield decreases as amount of poly(ethylene oxide) increases. This result shows that increasing the amount of poly(ethylene oxide) water equivalent gave fewer yields. This phenomenon can explain by the high reactivity and solubility of the POE in water compared to the aniline in particular at high temperature [61].
Proposed mechanism of polymerization and copolymerization
In the present study, the process of synthesis of PANI–Maghnite-H+ and its homologue PANI–PEO2000–Maghnite-H+ composites can be divided into the following three steps as shown in Figs. 16, 17, 18, 19, 20 and 21.
Proposed mechanism of homopolymer (PANI) catalyzed by Maghnite-H+ at 160 °C. 1 Initiation—the mechanism of the reaction occurs within the layers of the maghnite exchanged by H + protons, which are capable of initiating cationic polymerization. 2 Propagation—propagation is by the successive addition of monomers on the chain macromere growing. 3 Termination—the termination takes place during the recombination of two radical ions giving rise to the final polymer
Conclusion
Formation of PANI-PEO inside the maghnite (Algerian MMT) has been confirmed by HNMR spectroscopy. Infrared spectroscopy studies reveal the presence of physicochemical interaction, probably hydrogen bonding, between MMT, PANI and PEO. From the TGA result, it is observed that weight loss of PANI–MMT and PANI–PEO/MMT composite is less compared to pure PANI. In conclusion, PANI–maghnite and PANI–PEO2000–maghnite copolymers were successfully polymerized by chemical method under effect of ecological catalyst called Maghnite-H+ (Algerian MMT). In this present work, authors have carried out the green copolymer of polyaniline by poly(ethylene oxide) based on Maghnite-H+ (Algerian ecologic catalyst MMT) under microwave irradiation. This copolymer was prepared in order to combine the mechanical and physical properties of PEO2000 with conducting properties of PANI. The kinetics studies indicated that the polymerization and copolymerization rates are first order with respect to monomer concentration. A possible mechanism of this cationic polymerization is discussed based on the results of the 1H NMR. Spectroscopic analysis is of these model reactions. Thus, all the two types of composites provide opportunities and rewards creating new worldwide interest in these new materials.
References
Carini GD, Angelo G, Tripodo G, Bartolotta A, Di Marco G (1996) Low temperature excess specific heat and fragility in semicrystalline polymers. Phys Rev B 54:15056–15063
Brune DA, Bicerano J (2002) Micromechanics of nanocomposites comparison of tensile and compressive elastic moduli, and prediction of effects of incomplete exfoliation and imperfect alignment on modulus. Polymer 43(2):369–387
Mac Diarmid A (2001) Synthetic metals: a novel role for organic polymers. Angew Chem Int Edit 40:2581–2590
Rajesh AT, Kumar D (2009) Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sens Actuators B Chem 136:275–286
Zhang LJ, Long YZ, Chen ZJ, Wan MX (2004) The effect of hydrogen bonding on self assembled polyaniline nanostructures. Adv Funct Mater 14:693–698
Park S, Chung SW, Mirkin CA (2004) Hybrid organic–inorganic, rod-shaped nanoresistors and diodes. J Am Chem Soc 126:11772–11773
Jaroslav S, Irina S, Miroslava T (2010) Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog Polym Sci 35:1420
Zhang XY, Manohar SK (2004) Polyaniline nanofibers: chemical synthesis using surfactants. Chem Commun 20:2360–2361
Ray S, Okamoto M (2003) Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci 28(11):1539–1641
Rahmouni A, Harrane A, Belbachir M (2013) 1H-NMR spectra of conductive, anticorrosive and soluble polyaniline exchanged by an eco-catalyst layered (Maghnite-H+). Wod J Chem 8(1):20–26
Jang J (2006) Conducting polymer nonmaterials and their applications. Adv Polym Sci 199:189–259
Li X, Tian SJ, Ping Y, Kim DH, Knoll W (2005) One-step route to the fabrication of highly porous polyaniline nanofiber films by using PS-b-PVP diblock copolymers. As templates. Langmuir 21:9393–9397
Long YZ, Duvail JL, Wang QT, Li MM, Gu CZ (2009) Electronic transport through crossed conducting polymer nanowires. J Mater Res 24:3018–3022
Johnson SD, Anderson JM, Marchant RE (1992) Biocompatibility studies on plasma polymerized interface materials encompassing both hydrophobic and hydrophilic surfaces. J Biomed Mater Res 26:915–935
Raghunathan A, Kahol PK, Ho JC, Chen YY, Yao YD, Lin YS, Wessling B (1998) Low temperature heat capacities of polyaniline and polyaniline polymethylmethacrylate blends. Phys Rev B. 58:15955–15958
Belbachir M, Bensaoula A (2001) Composition and method for catalysis using bentonites. United States Patent Number: 6274527 B1
Belbachir M, Yahiaoui A, Hachemaoui A (2003) An acid exchanged montmorillonite clay-catalyzed synthesis of polyepichlorhydrin. Int J Mol Sci 4:548–561
Rahmouni A, Harrane A, Belbachir M (2012) Thermally stable forms of pure polyaniline catalyzed by an acid-exchanged MMT clay called maghnite-H+ as an effective catalyst. Int J Polymer Sci. https://doi.org/10.1155/2012/846710
Kumar KV, Rao UVS (2003) Thin Solid electrolyte systems based on PEO and their application as an electrochemical cell. In: Proceedings of 2nd international conference on ionic devices, 28–30th November Chennai, India
Choi HJ, Kim JW, Joo J, Kim BH (2001) Synthesis and electrorheology of emulsion intercalated PANI-clay nanocomposite. Synth Met 121(1–3):1325–1326
Kuilla T, Bhadra S, Yao DH, Kim NH, Bose S, Lee JH (2010) Recent advances in graphene based polymer composites. Prog Polym Sci 35:1350–1375
Spitalsky Z, Tasis D, Papagelis K, Galiotis C (2010) Carbon nanotube polymer composites: chemistry, processing, mechanical and electrical properties. Prog Polym Sci 35:357–401
Shin MK, Kim YJ, Kim SI, Kim SK, Lee H, Spinks GM, Kim SJ (2008) Enhanced conductivity of aligned PANi/PEO/MWNT nanofibers by electro-spinning. Sens Actuators B Chem 134:122–126
Pinto NJ, Johnson AT, Mueller CH, Theofylaktos N, Robinson DC, Miranda FA (2003) Electrospun polyaniline/polyethylene oxide nanofiber field-effect transistor. Appl Phys Lett 83:4244–4246
Yang Rui, Shichao Z, Lan Z, Wenbo L (2013) Electrical properties of composite polymer electrolytes based on PEO-SN-LiCF3SO3. Int J Electrochem Sci 8:10163
Devindrappa HU, Subba R, Ambika P (2006) Study of DC conductivity and battery application of PEO/PANI composites. J Power Sources 155:3689
Kunteppa H et al (2013) AC conductivity and battery application of polyethylene oxide/PANI/sodium chlorate composites. Adv Mater Lett 11:856
Gray KB, Rodrigues SJ (2001) Poly(ethyleneoxide)-based composite electrolytes: crystalline amorphous transition. J Electrochem Soc 148:1336
Sujeet KC, Rajendra KS (2010) Electrical conductivity studies on composite polymer electrolyte based on ionic liquid. Phase Transit 83:457
Olad A, Rashidzadeh A (2008) Preparation and anticorrosive properties of PANI/Na-MMT and PANI/OMMT nanocomposites. Prog Org Coat 622:93
Norris ID, Shaker MM, Ko FK, Macdiarmid AG (2000) Electrostatic fabrication of ultrafine conducting fibers: polyaniline/polyethylene oxide blends. Synth Met 114(2):109–114
Li Q, Cruz L, Phillips P (1993) Granular-rod model for electronic conduction in polyaniline. Phys Rev B 47:1840–1845
Wright PV (1975) Electrical conductivity in ionic complexes of poly(ethylene oxide). Br Polym J 7:319–327
Kherroub DE, Belbachir M, Lamouri S (2015) Nylon 6/clay nanocomposites prepared with Algerian modified clay (12-maghnite). Res Chem Intermed 41:5217–5228
Nascimento GM, Temperini ML (2008) Structure of polyaniline formed in different inorganic porous materials: a spectroscopic study. Eur Polym J 44:3501–3511
Zhang L, Zujovic ZD, Peng H, Bowmaker GA, Kilmartin PA, Travas-Sejdic J (2008) Structural characteristics of polyaniline nanotubes synthesized from different buffer solutions. Macromolecules 41:8877–8884
Rahmouni A, Harrane A, Belbachir M (2012) Kinetics study of catio-radically polymerization of aniline catalyzed by maghnite-Na clay catalyst layered (Western Algeria). World J Nano Sci Technol 1(2):26–32
Ayat M, Belbachir M, Rahmouni A (2016) Selective synthesis, characterization and kinetics studies of poly(α-Methyl styrene) induced by Maghnite-Na+ clay (Algerian MMT). Bull Chem React Eng Catal 11(3):376–388
Abdelkader R, Mohammed B (2016) Green synthesis of cationic polyacrylamide composite catalyzed by an ecologically catalyst clay called Maghnite-H+ (Algerian MMT) under microwave irradiation. Bull Chem React Eng Catal 11(2):170–175
Kao HM, Tsai YY, Chao SW (2005) Functionalized mesoporous silica MCM-41 in poly (ethylene oxide)-based polymer electrolytes: NMR and conductivity study. Solid State Ion 176:1261–1270
Xia JY, Qiua XP, Ma XM, Cui MZ, Yang J, Tang XZ, Zhu WT, Chen LQ (2005) Composite polymer electrolyte doped with mesoporous silica SBA-15 for lithium polymer battery. Solid State Ion 176:1249–1260
Xi JY, Tang XJ (2004) Enhanced lithium ion transference number and ionic conductivity of composite polymer electrolyte doped with organic–inorganic hybrid P123@SBA-15. Chem Phys Lett 400:68–73
Tominaga Y, Hong IC, Asai S, Sumita M (2007) Proton conduction in Nafion composite membranes filled with mesoporoussilica. J Power Sources 171:530–534
Gao Q, Xu Y, Wu D, Sun Y, Li XA (2009) PH-responsive drug release from polymer-coated mesoporous silica spheres. J Phys Chem C 113:12753–12758
Chu HQ, Yu C, Wan Y, Zhao DY (2009) Synthesis of ordered mesoporous bifunctional TiO2–SiO2-polymer nanocomposites. J Mater Chem 19:8610–8618
Praveen S, Sun ZF, Xu JG, Patel A, Wei Y, Ranade R, Baran G (2006) Compression and aging properties of experimental dental composites containing mesoporous silica as fillers. Mol Cryst Liq Cryst 448:223–231
Jiao J, Sun X, Pinnavaia TJ (2008) Reinforcement of a rubbery epoxy polymer by mesostructured silica and organo silica with wormhole framework structures. Adv Funct Mater 18:1067–1074
Darder M, Colilla M, Ruiz-Hitzky E (2003) Biopolymer–clay nanocomposites based on chitosan intercalated in montmorillonite. Chem Mater 15(20):3774–3780
Kassim A, Murali S, Adzmi F, Ekramul Mahmud EM (2004) Conducting polymer/clay composites: preparation and characterization. Mater Sci (Medziagotyra) 10(3):255–258
Tasneem A, Zahra R, Ahmad N (2011) Raman spectroscopy and AC conductivity of polynailine montmorillonite (PANI–MMT) nanocomposites. J Mater Sci Mater Electron 22(8):1076–1080
Ray SS, Bousima M (2005) Biodegradable polymers and their layered silicate nanocomposites: in greening the 21st century materials world. Prog Mater Sci 50:962–1079
Becker O, Varley RJ, Simon GP (2004) Thermal stability and water uptake of high performance epoxy layered silicate nanocomposites. Eur Polym J 40:187–195
Kalaleh H, Tally M, Atassi Y (2013) Preparation of a clay based superabsorbent polymer composite of copolymer poly (acrylate-co-acrylamide) with bentonite via microwave radiation. Res Rev Polym 4:145–150
Mohammad J, Zohuriaan M, Kourosh K (2008) superabsorbent polymer materials: a review. Iran Polym J 17:451–477
Okay O, Sarriisik SB (2000) Swelling behavior of poly(acrylamide-co-sodium acrylate) hydrogels in aqueous salt solutions: theory versus experiments. Eur Polym J 36:393–399
Pourjavadi A, Soleyman R, Bardajee GR (2009) Novel superabsorbent hydrogel based on natural hybrid backbone: optimized synthesis and its swelling behavior. Bull Korean Chem Soc 30:2680–2686
Danks TN (1999) Microwave assisted synthesis of pyrroles. Tetrahedron Lett 40:3957–3960
Ganji F, Vasheghani-Farahani S, Vasheghani-Farahani E (2010) Theoretical description of hydrogel swelling: a review. Iran Polym J 19:375–398
Sadeghi M, Hosseinzadeh H (2008) Synthesis and swelling behavior of starch-poly (sodium acrylate-co-acrylamide) superabsorbent hydrogel. Turk J Chem 32:375–388
Shi XN, Wang WB, Wang AQ (2011) Effect of surfactant on porosity and swelling behaviors of guar gum-g-poly(sodium acrylate-costyrene)/attapulgite superabsorbent hydrogels. Colloids Surf B Biointerfaces 88:279–286
Megherbi A, Meghabar R, Belbachir M (2013) Preparation and characterization of clay (Maghnite-H)/poly (3,4-ethylenedioxythiophene) composites. J. Surface Eng Mater Adv Technol 3:21–27
Acknowledgements
All our gratitude to the anonymous referees for their careful reading of the manuscript and valuable comments which helped in shaping this paper to the present form.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rahmouni, A., Belbachir, M. Molecular structure of PANI and its homologue PANI–PEO2000 catalyzed by Maghnite-H+ (Algerian MMT): synthesis, characterization and physical and chemical properties. Polym. Bull. 76, 4677–4701 (2019). https://doi.org/10.1007/s00289-018-2620-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2620-7