Abstract
New oligoetherols with 1,3,5-triazine ring were obtained from melamine–formaldehyde–butanone resins using 12 molar excess propylene oxide (with respect to the amount of introduced melamine). The resins were obtained from melamine solutions in reactive solvent obtained from simple chemical compound—methyl ethyl ketone (butan-2-one) in its reaction with 3 molar excess formaldehyde. Physical and chemical properties of obtained oligoetherols were determined and their structure was confirmed on the basis of proton magnetic resonance, infrared spectral data and MALDI-ToF mass analysis. The amount of by-products was specified on the basis of chromatographic analysis. Initial tests proved that obtained oligoetherols can constitute a potential substrate for obtaining polyurethane foams of increased thermal resistance.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The currently known methods of obtaining oligoetherols, which may constitute substrates for creating heat-resistant polyurethane foams, are in majority based on melamine reactions (Mel) isocyanuric acid (or their selected derivatives) with oxiranes or alkylene carbonates [1]. Insertion of azacyclic rings into the polymer structure is difficult due to weak solubility of their compounds in organic solvents.
One of such rings is (present in the melamine structure) 1,3,5-triazine (s-triazine) ring. Its presence results in high heat resistance of melamine and raises the temperature of decomposition to 360 °C. Introducing the 1,3,5-triazine ring into the polymer structure is impeded by melamine solubility, as it is weakly soluble only in dimethyl sulfoxide (DMSO) and water. So far, oligoetherols with the 1,3,5-triazine ring have been obtained in direct reactions of melamine and oxiranes (such as ethylene oxide or propylene oxide) conducted mostly in suspension of various solvents or the aforementioned DMSO [2–4].
The disadvantage of these methods was the necessity to distil toxic solvents after the reaction to separate oligoetherol. Moreover, DMSO used as the most popular melamine solvent would undergo thermal decomposition producing oligoetherol with unpleasant scent. Apart from this, using DMSO in industry encountered difficulties in the form of dissolving gaskets in pressurized reactors. The factors explain why effective anhydrous melamine solvents are still sought, as the presence of water causes creation of glycols and polyglycols as by-products undesirable in production of foamed polyurethane materials [5].
Such solvents may be the so-called reactive solvents, i.e. hydroxymethyl derivatives created in reactions of compounds built with active hydrogen atom [e.g. methyl ethyl ketone (MEK)] with formaldehyde [6]. Dissolution of melamine in reactive solvents leads to production of solutions being potential resources for synthesis of a new polycondensation polymer group with characteristics different from melamine to formaldehyde resins.
Research conducted in the Department of Organic Chemistry at the Rzeszow University of Technology led to obtaining solid materials with high water resistance, filled molding compounds and materials as well as heat-resistant foamed materials based on the reactive solvents (RS) obtained from hydroxymethyl derivatives of cyclohexanone [7] and methyl ethyl ketone [8–10]. In the recent years, a new group of oligoetherols was obtained, which was based on melamine solutions in reactive solvents subjected to reactions with oxiranes (ethylene and propylene oxides) in pressurized reactors. The results of the research are presented in this publication. In the synthesis, oxiranes (ethylene and propylene oxides) are used and the reactions are performed in pressurized reactors [11]. The literature does not contain any information on the use of the reactive solvents in the synthesis of oligoetherols with azacyclic rings except for one publication, where instead of melamine, isocyanuric acid was used [12].
This manuscript presents results of research concerning possibilities of obtaining new oligoetherols with 1,3,5-triazine ring based on polypropylene oxide (n Mel:n PO = 1:12) and melamine solutions in a reactive solvent obtained from methyl ethyl ketone (\(n_{\text{MEK}}:n_{{{\text{CH}}_{ 2} {\text{O}}}}\) = 1:3). Such oligoetherols might be a potential substrate for obtaining polyurethane foams with increased thermal stability.
Experimental
Chemical
Ethyl methyl ketone, Chempur, Poland; Formalin, Chempur, Poland; Triethylamine, Fluka, Switzerland; Melamine, Fluka, Switzerland; (±) Propylene oxide, Fluka, Switzerland.
Synthesis of reactive solvent
Ethyl methyl ketone (MEK), (64.8 g, 0.9 mol), formaldehyde (81.0 g, 2.7 mol), introduced in the form of formalin (275.2 g), as well as triethylamine (catalyst) in the amount necessary for pH 11, similar to the paper [13] were put into a three-neck flask of capacity 500 cm3 equipped with a reflux condenser and thermometer. Flask contents were mixed with a mechanical stirrer. After mixing the substrates, the reaction was carried out at 80 °C for 18 h. After the synthesis, the water and the catalyst were removed from the reaction environment by distillation under reduced pressure. To define the yield of the substrates in reaction of MEK and formaldehyde, mass balance of RS was established. Actual molar ratio \(n_{\text{MEK}}:n_{{{\text{CH}}_{ 2} {\text{O}}}}\) was calculated according to the formula:
where n MEK number of moles of methyl ethyl ketone (mol), n MEK number of moles of formaldehyde (mol), m MEK mass of methyl ethyl ketone used in reaction (g), M MEK molar mass of methyl ethyl ketone (g/mol), m RS mass of reactive solvent after distillation (g), and \(M_{{{\text{CH}}_{ 2} {\text{O}}}}\) molar mass of formaldehyde (g/mol).
A reactive solvent was obtained for which \(n_{\text{MEK}}:n_{{{\text{CH}}_{ 2} {\text{O}}}}\) = 1:2.7, with yield 94 wt%.
Melamine solubility in reactive solvent
The amount of melamine introduced into the reactive solvent (3-HMMEK) stemmed from previous studies [6, 13] and this was the maximal amount that did not cause gelation of the solution. 20.0, 36.7 and 46.1 g of melamine was introduced into 100 g of 3-HMMEK at the proportion of water (dosed during dissolving) 0, 10 and 20 wt%, respectively. Melamine–formaldehyde–butanone resins were obtained.
Obtaining of oligoetherols
3-HMMEK, melamine (in the amount that stemmed from solution tests) and water (in the amount, respectively, 0, 10 or 20 wt%) were introduced into a beaker of capacity 100 ml. The mixture was heated up to 90–100 °C, stirring with a glass rod until melamine has fully dissolved. Obtained melamine–formaldehyde–butanone resins was cooled down and put into a pressurized water reactor, where catalyst was also introduced (triethylamine (TEA)), as well as stirring element and an adequate amount of propylene oxide (PO). The reactor was closed with a stopper, provided with a heating–cooling jacket and placed on a magnetic stirrer. The mixture was heated up to 50–70 °C and kept in this condition until the oxirane has fully reacted. The reaction was considered as terminated when no loss of mass of post-reaction mixture was observed. Reaction products were orange or burgundy-brown resins.
The amount of PO necessary to obtain oligoetherols was calculated according to the formula:
where n Mel number of moles of melamine (mol), n PO number of moles of propylene oxide (mol), m Mel mass of melamine used in reaction (g), M Mel molar mass of melamine (g/mol), M PO mass of propylene oxide used in reaction (g), and M PO mass of propylene oxide (g/mol).
Physical and chemical properties
Physical properties of oligoetherols were evaluated in the temperature scope 20–80 °C. Density was determined using a pycnometer, viscosity with Höppler viscometer, surface tension by the detaching ring method and refractive index with the refractometric method (Abbe refractometer). The content of formaldehyde in oligoetherols permanently and removably bound was determined, respectively, with the use of sulphite and iodometric methods [14]. The hydroxyl number was determined according to the acylation method with acetic anhydride in the presence of pyridine [14].
Spectral analysis
1H-NMR spectra were carried out with the use of 1H-NMR spectrometer of operating frequency 500 MHz AVANCEII from BRUKER Biospin Company. d6-DMSO was used as the solvent and hexamethyldisiloxane (HMDSO) as the external standard. Recording was done in δ ppm. IR spectrum was carried out with the use of IR Paragon 1000 FT spectrometer from PERKIN ELMER Company by ATR technique.
Laser desorption/ionization (LDI) time-of-flight (ToF) mass spectrometry experiments were performed using a Bruker Autoflex Speed reflection time-of-flight mass spectrometer, equipped with a SmartBeam II laser (352 nm) in 80–1500 m/z range. The laser impulse energy was approximately 60–120 μJ, the laser repetition rate was 1000 Hz and the deflection value was set on m/z = 60. The first accelerating voltage was held at 19 kV and the second ion-source voltage was held at 16.7 kV. The reflector voltages used were 21 kV (first) and 9.55 kV (second). The data were recorded and analyzed using the software provided with the Autoflex instrument (FlexAnalysis version 3.3). Mass calibration typically cubic calibration based on five to seven points was performed using internal standards (gold ions and clusters from Au+ to Au10 + depending on m/z range). Sample solutions (ca. 5 mg/ml in CH3OH) were placed on AuNPET [15] (0.5 μL) with 0.5 μL standard DHB solution. Sum of ca. 7000 scans was collected for each sample.
Gas chromatography
Chromatographic analysis of oligoetherols was carried out with the use of internal standard (cyclohexanone). This method aimed to determine the presence of glycols in obtained products. Measurements were taken at the following recording conditions: initial temperature: 35 °C, heating rate: 20 °C/min, final temperature: 220 °C, time of heating at 220 °C: 6 min, dispenser temperature: 250 °C, and detector temperature: 300 °C. While preparing chromatograms of methanol solutions, as first appeared peak of cyclohexanone, and then expected by-products, that is glycols; propylene (PG), dipropylene (DPG) as well as tripropylene (TPG).
Results and discussion
Oligoetherols with 1,3,5-triazine ring were obtained from melamine–formaldehyde–butanone resin (Mel-F-MEK) and propylene oxide in the presence of alkaline catalyst. Mel-F-MEK resins were obtained from melamine solutions in a reactive solvent with the use of water (in the amount 0, 10 or 20 wt%) introduced during dissolving. All the reagents were placed in a pressurized reactor, and reactions were carried out at 50–70 °C (Table 1). The lower temperature range was restricted by too low speed of reaction, whereas the upper by the possibility of evaporation of low-boiling oxirane from the reaction environment. Oligoetherols were obtained as a result of reaction of 1 mol of melamine (in the form of solution in a reactive solvent) with 12 molar excess propylene oxide.
The product of reaction of 1 mol of methyl ethyl ketone (MEK) with 3 mol of formaldehyde was used as the reactive solvent (3-HMMEK) (see reaction below):

While choosing the reactive solvent as a substrate for obtaining oligoetherols, the following assumptions were made: the reactive solvent shall be obtained at the lowest possible molar relation MEK to formaldehyde, and the amount of dissolved melamine should be the highest possible, at the lowest possible share of water [10].
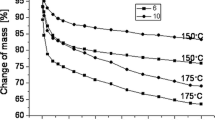
Given physical properties of obtained oligoetherols were determined, that is, density, viscosity, surface tension and refractive index (Table 2) and the influence of temperature on given properties of oligoetherols (anhydrous and obtained with the participation of water) was evaluated (Figs. 1, 2, 3, 4). A linear decrease of viscosity, surface tension, and refractive index as well as non-linear dependence on viscosity in the function of temperature were observed. The highest values of examined properties were observed for anhydrous oligoetherol and the lowest in case of oligoetherol obtained at 20 % of water introduced during dissolving of melamine. Sample physical values fall within the scope typical for polyols used for obtaining polyurethane foams. Surface tension is a decisive factor for the proper foaming process (that is, size of the pores and their uniformity) and viscosity influences foam stabilization.
Chemical properties of obtained oligoetherols were also evaluated, that is, the content of free and non-durably bound formaldehyde (respectively, with sulphite and iodometric method) and the hydroxyl number was set (Table 2). It was observed that irrespective of the amount of water introduced into RS during dissolving melamine, the amount of formaldehyde is close to zero, which means it has been fully blocked in reactions with oxirane. While determining the hydroxyl number, the content of hydroxy groups that appear in reactions between melamine and hydroxymethyl derivative (reaction 2) and then with propylene oxide (reaction 3) was evaluated.

During determination of hydroxyl number (L OH) of oligoetherols obtained at diverse share of water (introduced while dissolving melamine), it was observed that the highest value of the number (L OH = 375.4 mg KOH/g) can be seen in case of the product obtained at the highest share of water, whereas the lowest value—at the lowest share of water (L OH = 204.2 mg KOH/g) (Table 2). The result shows that in case of anhydrous oligoetherol, reactions of condensation take place that reduce the amount of reactive hydroxyl groups. Share of these reactions decreases with the increase of share of water introduced during dissolving melamine.
Structure of oligoetherols was confirmed on the basis of spectrum analysis of 1H-NMR and IR.
In 1H-NMR spectrum of anhydrous oligoetherol (Fig. 5), the presence of –CH2–CH(OH)–CH 3 protons signals can be observed, which indicates a regular opening of propylene oxide ring (in the scope 0.9–1.1 ppm). Product of abnormal ring opening is formed in trace levels (small signal of protons of –CH(CH 3)–CH2OH group at 1.1–1.2 ppm). Signal of methyl protons from starting ketone CH 3–CO– arises at chemical shift −2.1 ppm. The presence of methylene protons can be observed in the scope 3.1–3.6 ppm (respectively, –C–CH 2–OH, –O–CH 2–CH(CH3)–OH, –NH–CH 2–CH(CH3)–OH), and methine protons at chemical shift, respectively, 4.4 ppm –CH2–CH(OH)–CH3 and 4.5 ppm –CH(CH3)–CH2OH. The presence of hydroxyl group –O–CH2CH(OH)–CH3 can be observed at 4.6 ppm. Having added D2O to the sample, the signal goes low. Lack of signal of primary amino group –NH2 (at 5.9 ppm) proves its complete reaction with reactive solvent or/and propylene oxide.
In 1H-NMR spectra of oligoetherols obtained at 10 and 20 % of water introduced during dissolving melamine (Fig. 6) no significant changes in signals compared to the spectrum of anhydrous oligoetherol (Fig. 5) can be observed, apart from emergence of signal from water (at 3.2 ppm).
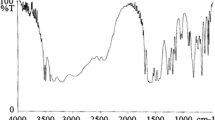
In IR spectrum of anhydrous oligoetherol (Fig. 7) one can observe the presence of strong and broad spectra of vibrations of associated hydroxyl groups (ν O–H, 3323 cm−1) and conjugated deforming vibrations of this group (δ O–H, 1373 cm−1), vibrations of methyl and methylene groups (\(v_{({\rm CH}_{2-}, {\rm CH}_{3-})}\), 2968–2875 cm−1) and strong vibrations of carbonyl group from starting ketone (ν C=O, 1699 cm−1). Moreover, there are vibration bands of ether groups (ν C–O–C, 1133 cm−1) as well as strong band of skeletal vibration of s-triazine ring (815 cm−1) confirming melamine integration into oligoetherol structure. In IR spectra of oligoetherols obtained with the use of water (introduced during dissolving of melamine), no significant differences in comparison with the spectrum of anhydrous oligoetherol were observed, with the exception of increased intensity of vibration bands from hydroxyl groups, which indicates that the number of these groups increases with the amount of water used to dissolve melamine.
The structure of oligoetherols is confirmed by MALDI-ToF mass spectra. Example spectrum of anhydrous oligoetherol is shown in Fig. 8. Basing on the spectrum analysis, it may be concluded that (irrespective of the amount of water introduced while dissolving melamine) mixtures of products of various degree of propylene oxide connection are obtained as oligoetherols (Table 3). On spectra, one can observe molecular peaks of m/z values corresponding to molecular weights of reaction products of following hydroxy groups with propylene oxide. MALDI-ToF analysis suggests that oligoetherols of various degrees of oxypropylene groups introduction arise, as in the spectrum, apart from peaks from raw materials (occurring at m/z values <160), Au+ ions (AuNPET plate [15]) as well as by-products—glycols (PG, DPG and TPG), one can observe molecular peaks different from each other by m/z units = 58 (in Table 3, e.g. sn. 51 and 53). MALDI-ToF mass analysis of anhydrous oligoetherol confirmed the presence of 12 mol of propylene oxide in the obtained product (Table 3).
On the basis of 1H-NMR and IR spectra as well as MALDI-ToF mass analysis, the structure of obtained oligoetherols was confirmed.
Obtained oligoetherols were subjected to chromatographic analysis (GC) to evaluate share of by-products created in the reaction of propylene oxide (PO) and water introduced to melamine–formaldehyde–butanone resins during dissolving of melamine or propylene oxide and condensing water (reaction 4). By-products are mainly glycols: propylene (PG), dipropylene (DPG) and tripropylene (TPG). DPG and TPG glycols are created as a result of consequent reaction of PG with, respectively, 1 and 2 mol of PO (reactions 5 and 6).

Share of by-products in oligoetherols is different, depending on the amount of water introduced during dissolving of melamine (Table 4). The lowest amount of glycols was observed in anhydrous oligoetherol, and the highest in the product obtained at 20 % share of water. Share of propylene glycol is the highest in anhydrous oligoetherol and decreases with increase of share of water (introduced while dissolving), which suggests that in case of anhydrous oligoetherol, the share of condensation reaction is the highest (Fig. 9). Higher share of water increases the probability of occurrence of consequent reactions of obtained propylene glycol with propylene oxide, which leads to increased share of dipropylene glycol (DPG) and tripropylene glycol (TPG) (Fig. 9).
Conclusion
Oligoetherols containing 1,3,5-triazine ring (being potential substrates for obtaining heat-resistant polyurethane foams) were obtained from melamine–formaldehyde–butanone resins using 12-molar excess propylene oxide (in relation to the amount of introduced water). Oligoetherols at 0, 10 and 20 % of water (introduced during dissolving of melamine) were obtained. The greater the amount of water used for dissolving, the greater is the amount of introduced melamine. Physical and chemical properties of obtained oligoetherols were examined. Lack of free formaldehyde in obtained products confirms its full blocking in reaction with oxirane. Oligoetherols structure was confirmed on the basis of 1H-NMR spectra, IR spectra as well as MALDI-ToF mass analysis. The amount of by-products was determined on the basis of chromatographic analysis. An increase in the amount of by-products was observed while increasing the amount of water introduced into melamine during its dissolution in a reactive solvent.
On the basis of the obtained results, one may presume that oligoetherols with 1,3,5-triazine ring based on melamine–formaldehyde–butanone resins might constitute a potential polyol factor for obtaining polyurethane foams of increased thermal resistance, which will be the subject of a separate publication.
References
Lubczak J (2003) Hydroxyalkylation of nitrogen-containing heterocyclic compounds. Curr Trends Polym Sci 8:73–105
Kucharski M, Lubczak J (1995) Method of obtaining polyetherols with s-triazine ring. Polish Patent No. 166 339
Kucharski M, Lubczak J (1991) Synthesis of polyetherols with s-triazine ring catalyzed by tetrabutylammonium hydroxide. Acta Polym 42:186–189
Lubczak J (2011) Polyhydroxyalkyl derivatives and polyetherols obtained from azacyclic compounds. Polym (Warsaw) 56:360–368
Wirpsza Z, Kucharski M, Lubczak J (1998) New melamine resins. I. Synthesis of reactive solvents for melamine. J Appl Polym Sci 67:1039–1049
Głowacz-Czerwonka D, Kucharski M (2005) New melamine-formaldehyde-ketone polymers: I. Synthesis of reactive solvents of melamine from selected ketones. J Appl Polym Sci 95:1319–1332
Głowacz-Czerwonka D, Kucharski M (2010) New melamine-formaldehyde-ketone resins. VI. Preparation of filled molding compositions and compression molding materials from reactive solvent based on cyclohexanone. J Appl Polym Sci 116:2802–2807
Głowacz-Czerwonka D, Kucharski M (2008) New melamine-formaldehyde-ketone polymers. V. The polymer coatings from reactive solvents obtained from ethyl-methyl ketone. J Appl Polym Sci 109:2156–2168
Głowacz-Czerwonka D (2013) Polyurethane foams from melamine solutions in reactive solvents based on methyl-ethyl ketone. J Appl Polym Sci 128:3465–3472
Głowacz-Czerwonka D (2013) Prospects of using melamine solutions in reactive solvents in polymer technology. CHEMIK 67:289–300
Głowacz-Czerwonka D, Lubczak J (2015) The method of obtaining polyetherols with 1,3,5-triazine ring. Polish Patent No. 221189
Wilk K, Lubczak J (2011) Application of reactive solvents of melamine for synthesis of polyetherols with perhydro-1,3,5-triazine rings. J Appl Polym Sci 119:776–785
Głowacz-Czerwonka D, Kucharski M (2006) New melamine–formaldehyde–ketone polymers. IV. Dissolution of melamine in reactive solvents prepared from methyl ethyl ketone. J Appl Polym Sci 100:1496–1505
Kasterina TN, Kalinina ŁS (1965) Chemical analysis of plastics materials. WNT-Ed, Warsaw (in Polish translated from Russian)
Sekuła J, Nizioł J, Rode W, Ruman T (2015) Gold nanoparticle-enhanced target (AuNPET) as universal solution for laser desorption/ionization mass spectrometry analysis and imaging of low molecular weight compounds. Anal Chim Acta 875:61–72
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Głowacz-Czerwonka, D. Oligoetherols based on melamine–formaldehyde–butanone resins. Polym. Bull. 74, 1743–1760 (2017). https://doi.org/10.1007/s00289-016-1802-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1802-4