Abstract
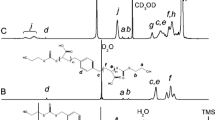
A novel glucose-containing ABC block terpolymer, poly(N-isopropyl acrylamide)-block-poly(acryloyl glucofuranose)-block-poly(acrylic acid) (PNAA) was synthesized by sequential reversible addition–fragmentation chain transfer polymerization. 2-Dodecylsulfanylthiocarbonylsulfanyl-2-methylpropionic acid was synthesized and was used as a chain transfer agent. The structures were characterized by Fourier transform infrared spectroscopy, proton nuclear magnetic resonance and gel permeation chromatography. Transmission electron microscopy and the phase transition behaviour confirmed that the block terpolymers could directly aggregate into spherical micelles. The results of dynamic light scattering and in vitro drug release experiments demonstrated that the content of the poly(3-O-acryloyl-glucofuranose) segments significantly influences the size of the micelles, the critical micelle concentration (CMC) and the drug release behaviours of the PNAA hydrogels. When the monomer molar ratios of acrylic acid/3-O-acryloyl-glucofuranose/N-isopropyl acrylamide are 4:2:14, the micelle size of PNAA is 292.3 nm, the CMC is the lowest (0.056 mg/mL), and the amount of cumulative drug release is up to 81 %. In vivo biocompatibility tests and in vitro cytotoxicity tests showed that the synthesized block terpolymer had no apparent cytotoxicity.













Similar content being viewed by others
References
Fan TF, Li MJ, Wu XM (2011) Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers. Colloids Surf B 8:593–600
Reza MG, Zeinab R, Shiva K (2014) Magnetic/pH-sensitive kappa-carrageenan/sodium alginate hydrogel nanocomposite beads: preparation, swelling behavior, and drug delivery. J Biomat Sci Polym E 25:1891–1906
Michele LTK, Ernandes TNT, Marcos GR (2015) Water transport properties through starch-based hydrogel nanocomposites responding to both pH and a remote magnetic field. Chem Eng J 259:620–629
Hassan S, Soheila S, Shohreh A (2015) Preparation and application of magnetic graphene oxide coated with a modified chitosan pH-sensitive hydrogel: an efficient biocompatible adsorbent for catechin. RSC Adv 5:9396–9404
Huang GX, Zhu J, Zhang ZB, Zhang W, Zhou NC, Zhu XL (2013) Reversible photo- and thermo-responsive block copolymer micelles functionalized by NIPAM and azobenzene. J Macromol Sci A 50:193–199
Kim JII, Chun C, Kim B, Hong JM, Cho JK, Lee SH, Song SC (2012) Thermosensitive/magnetic poly(organophosphazene) hydrogel as a long-term magnetic resonance contrast platform. Biomaterials 33:218–224
Ma LW, Liu MZ, Liu HL (2010) In vitro cytotoxicity and drug release properties of pH- and temperature-sensitive core-shell hydrogel microspheres. Int J Pharmaceut. 385:86–91
Schilli CM, Zhang MF, Rizzardo E, Thang SH, Chong YK, Edwards K, Karlsson G, Müller AHE (2004) A new double-responsive block copolymer synthesized via RAFT polymerization: poly(N-isopropylacrylamide)-block-poly(acrylic acid). Macromolecules 37:7861–7866
Dzinomwa GPT, Wood CJ, Hill DJT (1997) Fine coal dewatering using pH and temperature-sensitive superabsorbent polymers. Polym Advan Technol 8:767–772
Heo J, Thomas KJ, Seong GH (2003) A microfluidic bioreactor based on hydrogel-entrapped E. coli: cell viability, lysis, and intracellular enzyme reactions. Anal Chem 75:22–26
Aleksander S, Shameema SF, Aurelie C (2010) The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. Biomaterials 31:8426–8435
Rivero RE, Molina MA, Rivarola CR, Barbero CA (2014) Pressure and microwave sensors/actuators based on smart hydrogel/conductive polymer nanocomposite. Sens Actuators B Chem 190:270–278
Hava O, Ozgur O (2013) Rhodamine based reusable and colorimetric naked-eye hydrogel sensors for Fe3+ ion. Chem Eng J 232:364–371
Mano JF, Silva GA, Azevedo HS (2007) Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 4:999–1030
Oommen OP, Wang SJ, Kisiel M (2013) Smart design of stable extracellular matrix mimetic hydrogel: synthesis, characterization, and in vitro and in vivo evaluation for tissue engineering. Adv Funct Mater 23:1273–1280
Hynd MR, Turner JN, Shain W, Biomat J (2009) Applications of hydrogels for neural cell engineering. Sci Polym E 18:1223–1244
Hatefi A, Amsden B (2002) Biodegradable injectable in situ forming drug delivery systems. J Control Release 80:9–28
Thomas K, Li YX, Florian U (2002) ABA-triblock copolymers from biodegradable polyester A-blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins. Adv drug deliver rev 54:99–134
Bajpai AK, Shukla SK, Bhanu S (2008) Responsive polymers in controlled drug delivery. Prog Polym Sci 33:1088–1118
Heskins M, Guillet JE (1968) Solution properties of poly (N-isopropylacrylamide). J Macromol Sci Chem A 2:1441–1455
Xia Y, Yin XC, Burke NAD, Stover HDH (2005) Thermal response of narrow disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 38:5937–5943
Lowe AB, McCormick CL (2007) Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under homogeneous conditions in organic and aqueous media. Prog Polym Sci 32:283–351
Bulmus V, Ding Z, Long CJ, Stayton PS, Hoffman AS (2000) Site-specific polymer–streptavidin bioconjugate for pH-controlled binding and triggered release of biotin. Bioconjug Chem 11:78–83
Yin X, Hoffman AS, Stayton PS (2006) Poly (N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 7:1381–1385
Moad G, Rizzardo E, Thang SH (2005) Living radical polymerization by the RAFT process. Aust J Chem 58:379–410
Koßmehl G, Volkheime J (1989) Synthesis of polymerizable hexose derivatives. Liebigs Ann Chem 11:1127–1130
Lai JT, Filla D, Shea R (2002) Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents. Macromolecules 35:6754–6756
Li H, Sivasankarapillai G, McDonald AG (2015) Highly biobased thermally stimulated shape memory copolymeric elastomers derived from lignin and glycerol-adipic acid based hyperbranched prepolymer. Ind Crop Prod 67:143–154
Li H, Sivasankarapillai G, McDonald AG (2014) Lignin valorization by forming thermally stimulated shape memory copolymeric elastomers-partially crystalline hyperbranched polymer as crosslinks. J Appl Polym Sci 131:41103
Sun TM, Zhu JL, Wang M, Lu ML, Ding JJ, Lv ZT, Hua P, Zhang YJ (2015) A glucosyl triblock copolymer: synthesis and its injectable thermo- and pH-responsive behaviours. RSC Adv 5:24231–24238
Tang YF, Zhang SM, Wang M, Zhu JL, Sun TM, Jiang GQ (2014) A glucose-based diblock copolymer: synthesis, characterization and its injectable/temperature-sensitive behaviors. J Polym Res 21:1–8
Peng CL, Shieh MJ, Tsai MH, Chang CC, Lai PS (2008) Self-assembled star-shaped chlorin-core poly(ɛ-caprolactone)–poly(ethylene glycol) diblock copolymer micelles for dual chemo-photodynamic therapies. Biomaterials 29:3599–3608
He YY, Zhang Y, Xiao Y, Lang MD (2010) Dual-response nanocarrier based on graft copolymer with hydrazine bond linkages for improved drug delivery. Colloids Surf B Bioing 80:145–154
Abandansari HS, Aghaghafari E, Nabid MR, Niknejad H (2013) Preparation of injectable and thermoresponsive hydrogel based on penta-block copolymer with improved sol stability and mechanical properties. Polymer 54:1329–1340
Acknowledgments
This work was sponsored by the National Natural Science Foundation of China (Nos. 21476117, 21376124, 21303090), the Science and Technology Projects Fund of Nantong City (BK2014014) and Jiangsu Province “Qing Lan Project”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Lu, M., Wang, M. et al. A biocompatible glucose-containing ABC block terpolymer: synthesis, characterization and its properties in solution. Polym. Bull. 73, 2373–2390 (2016). https://doi.org/10.1007/s00289-016-1711-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1711-6