Abstract
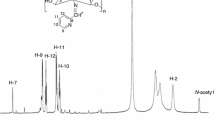
The chitosan Schiff bases were synthesised through the condensation reaction of chitosan with o-, m- and p-nitrobenzaldehyde (abbreviated as CSB-o, CSB-m and CSB-p) in the ratio 1:1 and were characterised by means of FTIR, UV, XRD and SEM. The thermal dehydration and degradation kinetics of all these Schiff bases were studied using different isoconversional and maximum rate (peak) methods, viz. Kissinger–Akahira–Sunose (KAS), Tang, Starink, Flynn–Wall–Ozawa (FWO) and Bosewell from DSC data and the thermal stability from TG. The activation energy values of thermal dehydration and degradation reactions obtained from isoconversional methods of FWO and Bosewell are slightly higher than that obtained from other methods. All the isoconversional and maximum rate (peak) methods yielded consistent values of E α for both the dehydration and degradation reactions and is in the order CSB-o < CSB-m < CSB-p. The Schiff bases observed (from TG) the same order of thermal stability.








Similar content being viewed by others
References
Crini G (2005) Recent developments in polysaccharide based materials used as adsorbents in waste water treatment. Prog Polym Sci 30:38–70
Agullo E, Rodtiquez MS, Ramos V, Albertengo L (2003) Present and future role of chitin and chitosan in food. Macromol Biosci 3:521–530
Sashiwa H, Aiba SI (2004) Chemically modified chitin and chitosan biomaterials. Prog Polym Sci 29:887–893
Chenite A, Chaput C, Wing D, Combes C, Buschmann MD, Hoemann CD, Leroax JC, Atkinso BL, Binelte F, Selmani A (2000) Novel injectable neutral solution of chitosan from biodegradable gels in situ. Biomaterials 21:2155–2161
Hsu SH, Whu SW, Tsai CL, Wu YH, Chen HW, Hsieh KH (2004) Chitosan as scaffold materials, effect of molecular weight and degree of deacetylation. J Polym Res 11:141–147
Huang M, Khor E, Lim LY (2004) Chitosan uptake and cytotoxicity of molecules and nanoparticles: effect of molecular weight and degree of deacetylation. Pharm Res 21:344–353
Binsu VV, Nagarate RK, Shahi VK, Ghosh PK (2006) Studies on N-methylene phosphonic acid chitosan/poly (vinyl alcohol) composite proton exchange membranes. React Funct Polym 66:1619–1629
Britto D, Assis OBG (2006) A novel method for obtaining a quaternary salt of chitosan. Carbohydr Polym 69:305–310
Prasanth HKV, Tharanathan RN (2007) Chitin/Chitosan modification and their unlimited application potential. Trends Food Sci Technol 18:117–131
Xu WL, Liu JD, Sun Chin YP (2003) Preparation of cyclomaltoheptanose from chitosan and its derivative via glyoxal and glutaraldehyde. Chem Lett 14:767–770
Lim SH, Hudson SM (2004) Synthesis and antimicrobial activity of water soluble chitosan derivative with a fiber reactive group. Carbohydr Res 339:313–319
Santos JE, Dockal ER, Cavalheiro ETG (2005) Synthesis and characterization of Schiff bases from chitosan and salicylaldehyde derivatives. Carbohydr Polym 60:277–282
Cimerman Z, Galic N, Bosener B (1997) The Schiff bases of salicylaldehyde and aminopyridines as highly sensitive analytical reagents. Anal Chim Acta 343:145–153
Hall LD, Yalpani M (1980) Enhancement of the metal chelating properties of chitin and chitosan. Carbohydr Res 83:5–7
Rodrigues CA, Laranjeira MCM, de-Favere VT, Sadler E (1998) Interaction of Cu(II) on N-(2-pyridylmethyl) and N-(4-pyridylmethyl) chitosan. Polymer 39:5121–5129
Kurita K, Mori S, Nishiyama Y, Harata M (2002) N-Alkylation of chitin and some characteristics of novel derivatives. Polym Bull 48:159–166
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Wu F, Tseng R, Juang R (2010) A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J Environ Manage 91:798–806
Wang FY, Wang H, Ma JW (2010) Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J Hazard Mat 177:300–306
Peniche C, Carlos E, Roman JS (1998) Interpolymer complexes of chitosan and polymethacrylic derivatives of salicylic acid: preparation, characterization and modification by thermal treatment. Polymer 39:6549–6554
Velyana G, Dilyana Z, Lyubomir V (2012) Non-isothermal kinetics of thermal degradation of chitosan. Chem Central J 6:81–91
de Douglas B, Sergio Paulo C (2007) Kinetics of the thermal degradation of chitosan. Thermochim Acta 465:73–82
Shen-Kun L, Chi-Chih H, Ming-Fung L (2004) A kinetic study of thermal degradations of chitosan/polycaprolactam blends. Macromol Res 12:466–473
Tirkistani FAA (1998) Thermal analysis of some chitosan Schiff bases. Polym Degrad Stab 60:67–70
Ikejima T, Yogi K, Inonu Y (1999) Thermal properties and crystallization behavior of poly(3-hydroxybutyric acid) in blends with chitin and chitosan. Macromol Chem Phys 200:413–421
Chun-Yan O, Chao-Hua Z, Si-Dong L, Lei Y, Jing-Jing D, Xue-Liu M, Mu-Ting Z (2010) Thermal degradation kinetics of chitosan-cobalt complex as studied by thermogravimetric analysis. Carbohydr Polym 82:1284–1289
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Kittur FS, Harish PKV, Sankar KU, Tharanathan RN (2002) Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr Polym 49:185–193
Vyazovkin S (2000) Computational aspects of kinetic analysis, Part C. The ICTAC Kinetics Project—the light at the end of the tunnel? Thermochim Acta 355:155–163
Vyazovkin S, Sbirrazzuoli N (2002) Isoconversional analysis of the non-isothermal crystallization of a polymer melt. Macromol Rapid Comm 23:766–770
Vyazovkin S, Sbirrazzuoli N (2003) Estimating the activation energy for non-isothermal crystallization of polymer melts. J Therm Anal Calorim 72:681–686
Joraid AA, Abu-Sehly AA, El-Oyoun MA, Alamri SN (2008) Non-isothermal crystallization kinetics of amorphous Te51.3As45.7Cu3. Thermochim Acta 470:98–104
Starink MJ (2003) The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta 404:163–176
Vyazovkin S, Sbirrazzuoli N (2004) Isoconversional approach to evaluating the Hoffman–Lauritzen parameters (U* and Kg) from the overall rates of non-isothermal crystallization. Macromol Rapid Comm 25:733–738
Khawam A, Flanagan DR (2005) Role of isoconversional methods in varying activation energies of solid-state kinetics: II. Non-isothermal kinetic studies. Thermochim Acta 436:101–112
Vyazovkin S (2006) Model-free kinetics, staying free of multiplying entities without necessity. J Therm Anal Calorim 83:45–51
Vyazovkin S, Sbirrazzuoli N (2006) Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Comm 27:1515–1532
Starink MJ (2007) Activation energy determination for linear heating experiments: deviations due to neglecting the low temperature end of the temperature integral. J Mater Sci 42:483–489
Vyazovkin S, Wight CA (1998) Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int Rev Phys Chem 17:407–433
Bonnet E, White RL (1998) Species-specific isoconversion effective activation energies derived by thermogravimetry-mass spectrometry. Thermochim Acta 311:81–86
Muraleedharan K, Kripa S (2014) Thermal dehydration kinetics of potassium bis(oxalato)cuprate(II)dehydrate. J Anal Appl Pyrol 107:298–305
Kissinger HE (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Nat Bur Stand 57:217–221
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics committee recommendations for performing kinetic computations on thermal analysis data-Review. Thermochim Acta 520:1–19
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886
Rao CNR (1963) Chemical applications of infra red spectroscopy. Academic Press, New York, pp 365–366
Tang W, Liu Y, Zhang H, Wang C (2003) New approximate formula for Arrhenius temperature integral. Thermochim Acta 408:39–43
Doyle C (1961) Kinetic analysis of thermogravimetric data. J Appl Polym Sci 5:285–292
Starink MJ, Van Mourik P (1992) Cooling and heating rate dependence of precipitation in an Al-Cu alloy. Mater Sci Eng A 156:183–194
Starink MJ (1997) On the applicability of isoconversion methods for obtaining the activation energy of reactions within a temperature dependent equilibrium state. J Mater Sci 32:6505–6512
Guo Z, Xing R, Liu S, Zhong Z, Ji X, Wang L, Li P (2007) Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr Res 342:1329–1332
Issa RM, Khedr AM, Rizk HF (2005) UV-Vis, R, 1HNMR Spectroscopic studies of some Schiff bases derivative of 4-aminoantipyrine. Spectrochim Acta 62:621–629
Jin X, Wang J, Bai J (2009) Synthesis and antimicrobial activity of the Schiff base from chitosan and citral. Carbohydr Res 344:825–829
Focher B, Beltranme PL, Naggi A, Torri G (1990) Alkaline deacetylation of chitin enhanced by flash treatments: reaction kinetics and structure modifications. Carbohydr Polym 12:405–418
Zhang Y, Xue C, Xue Y, Gao R, Zhang X (2005) Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr Res 340:1914–1917
Kumirska J, Czerwicka M, Kaczynski Z, Bychowska A, Brzozowski K, Thöming J, Stepnowski P (2010) Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar Drugs 8:1567–1636
Jarzabek B, Kaczmarczyk B, Sek D (2009) Characteristic and spectroscopic properties of the Schiff base model compounds. Spectrochim Acta 74:949–954
Anan NA, Hassan SM, Saad EM, Buutler IS, Mostafa SI (2011) Preparation, characterization and pH metric measurements of 4-hydroxysalicylidinechitosan Schiff base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Ru(III), Rh(III), Pd(II) and Au(III). Carbohydr Res 346:775–793
Kimura S, Isobe N, Wda M, Kuga S, Ko JH, Kim UJ (2011) Enzymatic hydrolysis of chitosan-dialdehyde cellulose hydrogels. Carbohydr Polym 83:1850–1853
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muraleedharan, K., Viswalekshmi, C.H. & Sarada, K. Synthesis, characterization and thermal dehydration and degradation kinetics of chitosan Schiff bases of o-, m- and p-nitrobenzaldehyde. Polym. Bull. 74, 39–54 (2017). https://doi.org/10.1007/s00289-016-1696-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1696-1