Abstract
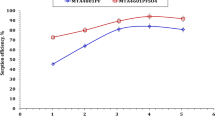
A novel terpolymer acts as an effective chelating ion exchanger which was synthesized using 2-amino-6-nitro-benzothiazole and semicarbazide with formaldehyde (BSF) by solution condensation technique. Its ion exchange properties was determined against certain metal ions viz. Fe3+, Co2+, Ni2+, Cu2+, Zn2+ and Pb2+ using batch equilibrium technique with different electrolyte concentrations, pH ranges and time intervals. The results of batch studies revealed that the separation of the selected metal ions from the aqueous solution by the terpolymer is found to be excellent compared to the available commercial resins and earlier reported resins. The order of metal ion uptake at higher concentrations by the BSF terpolymer at lower pH is Cu2+ > Ni2+ > Fe3+ and at lower concentration at higher pH is Zn2+ > Co2+ > Pb2+. The reusability of the resin was also reported for its effective ion-exchange behaviour for several cycles. The adsorption isotherm model was evaluated and the results are in good agreement with each other. The order of kinetics was also determined and the resin follows pseudo-second-order kinetics. Moreover, the physico-chemical analysis gives strong evidence for the effective metal ion removal compared with the earlier reported and commercial resins. Earlier, the structure and the properties of the synthesized novel chelating resin were clearly elucidated by elemental, FTIR, UV–Vis, 1H & 13C NMR spectra, GPC, SEM and XRD.













Similar content being viewed by others
References
Bagla P, Kaiser J (1996) India’s Spreading Health Crisis Draws Global Arsenic Experts. Science 274:174–175
Lepkowski W (1998) Arsenic crisis in Bangladesh, C&EN Washington. In: Chemical and Engineering News. November 16, pp 27–29
Clayton GD, Clayton FE (1994) Patty’s industrial hygiene and toxicology, vol 2C. Wiley-Interscience, New York
Lee MS, Ahn JG, Ahn JW (2005) A novel amine-shielded surface cross-linking of chitosan hydrogel beads for enhanced metal adsorption performance. Ind Eng Chem Res 44:6692–6700
Starvin AM, Prasada Rao T (2004) Removal and recovery of mercury(II) from hazardous wastes using 1-(2-thiazolylazo)-2-naphtol functionalized activated carbon as solid phase extractant. J Hazard Mater 113:75–79
Chandra D, Yokoi T, Tatsumi T, Bhaumik A (2007) Highly luminescent organic-inorganic hybrid mesoporous silicas containing tunable chemosensor inside the pore wall. Chem Mater 19:5347–5354
Chandra D, Kumar Das S, Bhaumik A (2010) A fluorophore grafted 2D-hexagonal mesoporous organosilica: excellent ion exchanger for the removal of heavy metal ions from wastewater. Microporous Mesoporous Mater 128:34–40
Hodgkin JH, Mark HF, Bikales NM, Overberger CG, Menges G (eds) (1985) Chelate-forming polymers. Encyclopedia of Polymer Science and Engineering, vol. 3. Wiley, New York, pp 363–381
Qu RJ, Ji CN, Sun YZ, Li ZF, Cheng GX, Song RF (2004) Syntheses and adsorption properties of phenol-formaldehyde-type chelating resins bearing the functional group of tartaric acid. Chin J Polym Sci 22(5):469–475
Jadhao MM, Paliwal LJ, Bhave NS (2005) Resin I: Synthesis and characterization of 2,2′-dihydroxybiphenyl–urea–formaldehyde terpolymers. J Appl Polym Sci 96:1605–1610
Rahangdale SS (2012) Metal ion binding and biological properties of copolymer resin derived from 2, 4-Dihydroxyacetophenone. Arch Appl Sci Res 4(5):2280–2288
Gurnule WB, Patle DB (2010) Study of chelation ion-exchange properties of new copolymer resin derived from o-aminophenol, urea and formaldehyde. Arch Appl Sci Res 2(1):261–276
Karunakaran M, Magesh C (2010) Thermal and ion-exchange studies on chelating terpolymer resins derived from o cresol urea formaldehyde. Arab J Chem. doi:10.1016/j.arabjc.2010.06.057
Kırcı S, Gulfen M, Aydın AO (2009) Separation and recovery of silver(I) ions from base metal ions by thiourea-or urea–formaldehyde chelating resin. Sep Sci Technol 44(8):1869–1883
Jadhao MM, Paliwal LJ, Bhave NS (2009) Ion-exchange properties of 2,2′-dihydroxybiphenyl-urea-formaldehyde terpolymer resins. Desalination 247:456–465
Gurnule WB, Rahangdale PK, Paliwal LJ, Kharat RB (2003) Chelation ion–exchange properties of copolymer resins derived from 2-hydroxyacetophenone, oxamide and formaldehyde. Synth React Inorg Met Org Chem 33(7):1187–1205
Gurnule WB, Rahangdale PK, Paliwal LJ, Kharat RB (2002) Chelation ion-exchange properties of copolymer derived from 4-hydroxyacetophenone, oxamide and formaldehyde. Prog Cryst Growth Charact Mater 45:127–132
Gurnule WB, Butoliya SS, Zade AB (2009) Terpolymer resin VIII: chelation ion exchanger properties of 2,4-dihydroxybenzophenone-oxamide-formaldehyde terpolymer resins. J Appl Polym Sci 113:1–9
Singru RN, Gurnule WB (2010) Chelation ion-exchange properties of copolymer resins derived from p-cresol, oxamide and formaldehyde. Iran Polym J 19(3):169–189
Gurnule WB, Rahangdale PK, Paliwal LJ, Kharat RB (2003) Synthesis, characterization and ion-exchange properties of 4-hydroxyacetophenone, biuret and formaldehyde terpolymer resins. React Funct Polym 55:255–265
Shah BA, Shah AV, Navik AJ (2012) Microwave-promoted M-PFR resin: kinetics, isotherms and sequestration of Cr(III) and Pb(II) from environmental samples. Iran Polym J 21(9):569–582
Azarudeen R, Ahamed MR, Arunkumar P, Prabu N, Jeyakumar D, Burkanudeen A (2010) Metal sorption studies of a novel terpolymer resin. Int J Chem Environ Eng 1(1):23–28
Azarudeen RS, Ahamed MAR, Jeyakumar D, Burkanudeen AR (2009) An eco-friendly synthesis of a terpolymer resin: Characterization and chelation ion-exchange property. Iran Polym J 18(10):821–832
Ahamed MR, Azarudeen R, Karunakaran M, Karikalan T, Manikandan R, Burkanudeen A (2010) Cation exchange properties of a terpolymer: synthesis and characterization. Int J Chem Environ Eng 1(1):7–12
Vogel A (1989) Text book of quantitative chemical analysis, 5th edn. Longman, UK, pp 186–213
Gregor HP, Taifer M, Citarel L, Becker EI (1952) Chelate ion exchange resins. Ind Eng Chem 44:2834–2839
Ahamed MAR, Azarudeen RS, Karunakaran M, Burkanudeen AR (2010) Synthesis, characterization, metal ion binding capacities and applications of a terpolymer resin of anthranilic acid/salicylic acid/formaldehyde. Iran Polym J 19(8):635–646
Lingala PS, Paliwal LJ, Juneja HD (2001) Synthesis and characterization of 8-hydroxyquinoline-oxamide-formaldehyde terpolymer. Proc Nat Acad Sci India 71:205–212
Nakamoto K, Mccarthy PJ (1986) Spectroscopy and structure of metal chelate compounds. Wiley, New York, p 296
Ahamed MAR, Burkanudeen RS (2012) Metal complexes of a novel terpolymer ligand: synthesis, spectral, morphology, thermal degradation kinetics and antimicrobial screening. J Inorg Organomet Polym 22:1046–1061
Williams DH, Fleming E (1975) Spectroscopy methods in organic chemistry, 4th edn. Tata McGraw Hill, UK
Kemp W (1996) Organic spectroscopy, 3rd edn. The Macmillan Press Ltd, Hong Kong
Pretsch E, Buhlmann P, Afflolter C (2000) Structure determination of organic compounds. Springer, New York
Ahamed MAR, Azarudeen RS, Jeyakumar D, Burkanudeen AR (2011) Terpolymer chelates: synthesis, characterization, and biological applications. Int J Polym Mater 60:124–143
Nanjundan S, Jone Selvamalar CS, Jayakumar R (2004) Synthesis and characterization of poly(3-acetyl-4-hydroxyphenyl acrylate) and its Cu(II) and Ni(II) complexes. Eur Polym J 40:2313–2321
Das SC (2000) Ion exchange studies of resin copolymer derived from aromatic hydroxyl compounds. J Indian Chem Soc 77:66–69
Azarudeen RS, Ahamed MAR, Burkanudeen AR (2011) Chelating terpolymer resin: synthesis, characterization and its ion-exchange properties. Desalination 268:90–96
Kapadia M, Patel M, Joshi J (2008) Synthesis of phenolic resin and its polychelates with 4f-block elements: thermal and adsorption studies. Iran Polym J 17(10):767–779
Azarudeen RS, Burkanudeen AR (2012) Sorption investigation on the removal of metal ions from aqueous solutions using chelating terpolymer resin. Res Chem Intermed 38:2155–2173
Azarudeen RS, Subha R, Jeyakumar D, Burkanudeen AR (2013) Batch separation studies for the removal of heavy metal ions using a chelating terpolymer: synthesis, characterization and isotherm models. Sep Purif Technol 116:366–377
Subha R, Namasivayam C (2009) Kinetics and isotherm studies for the adsorption of phenol using low cost micro porous ZnCl2 activated coir pith carbon. Can J Civil Eng 36:148–159
Treybal RE (1980) Mass transfer operations. McGraw Hill, New York
Acknowledgments
The authors thank the Management and Principal of Oxford Engineering College, Tiruchirappalli, Coimbatore Institute of Technology, Coimbatore and Jamal Mohamed College (Autonomous), Tiruchirappalli, Tamil Nadu for their support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riswan Ahamed, M.A., Azarudeen, R.S., Subha, R. et al. Sorption behavior of ion-exchange terpolymer resin with environmental impact: synthesis, characterization and isotherm models. Polym. Bull. 71, 3209–3235 (2014). https://doi.org/10.1007/s00289-014-1246-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1246-7