Abstract
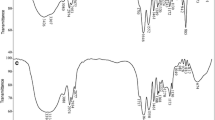
Recently, a renewed interest in hydrogels for heavy metal removal of wastewater has been growing because of embarking opportunities in industrial applications. One of the most interesting hydrogels potentially used as absorbent is poly(vinyl alcohol) (PVA), owing to its biocompatibility. In this study, the adsorption capacity of copper(II) ion onto PVA hydrogel (PVAH) adsorbents with different crosslinking degrees of 1, 3 and 5 % from aqueous solution was investigated. The PVAH adsorbents were prepared from PVA, using glutaraldehyde as a crosslinking agent. Their properties were determined by Fourier transform infrared spectroscopy, scanning electron microscopy (SEM), and water absorption measurement. The results showed that PVA was crosslinked with glutaraldehyde. It exhibited an equilibrium swelling ratio in the range of 195–250 %, depending on the crosslinking degree with different PVAH structures defined from SEM micrographs. The adsorption capacity of copper(II) ion onto PVAH adsorbents was investigated and found that higher crosslinking degree decreased the absorption capacity. This behavior is due to the decrease in reactive sites, resulting in the decrease of interaction between copper(II) ion and PVA. Besides, the adsorption capacity also depended on contact time, pH and temperature. The adsorption process followed pseudo-second-order kinetic, having a 0.99 correlation coefficient. Intraparticle diffusion was confirmed by the adsorption mechanism controlled by particle and film diffusions.














Similar content being viewed by others
References
Ngah WSW, Kamari A, Koay YJ (2004) Equilibrium kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int J Biol Macromol 34:155–161
Chen JH, Lin H, Luo ZH, He YS, Li GP (2011) Cu (II)-imprinted porous film adsorbent Cu–PVA–SA has high uptake capacity for removal of Cu (II) ions from aqueous solution. Desalination 277:265–273
Lin SH, Juang RS (2002) Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J Hazard Mater 92:315–326
Bhattacharya AK, Mandal SN, Das SK (2006) Adsorption of Zn (II) from aqueous solution by using different adsorbents. Chem Eng J 123:43–51
Wan MW, Kan CC, Rogel BD, Dalida MLP (2010) Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohyd Polym 80:891–899
Gyliene O, Visniakova S (2008) Heavy metal removal from solutions using natural and synthetic sorbents. Environ Res Eng Manage 43:28–34
Li X, Li Y, Ye Z (2011) Preparation of macroporous bead adsorbents based on poly(vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chem Eng J 178:60–68
Mohan D, Singh KP (2002) Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse an agricultural waste. Water Res 36:2304–2318
Futalan CM, Kan CC, Dalida ML, Hsien KJ, Pascua C, Wan MW (2011) Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohyd Polym 83:528–536
Khoo KM, Ting YP (2001) Biosorption of gold by immobilized fungal biomass. Biochem Eng J 8:51–59
Kao WC, Wu JY, Chang CC, Chang JS (2009) Cadmium biosorption by poly(vinyl alcohol) immobilized recombinant Escherichia coli. J Hazard Mater 169:651–658
Jamnongkan T, Kaewpirom S (2010) Potassium release kinetics and water retention of controlled-release fertilizers based on chitosan hydrogels. J Polym Environ 18:413–421
Matsumura S, Tomizawa N, Toki A, Nishikawa K, Toshima K (1999) Novel poly(vinyl alcohol)-degrading enzyme and the degradation mechanism. Macromolecules 32:7753–7761
Mansur HS, Costa HS (2008) Nanostructured poly(vinyl alcohol)/bioactive glass and poly(vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applications. Chem Eng J 137:72–83
Street KW, Hill CM, Philipp WH (2004) Properties of a novel ion-exchange film. Ind Eng Chem Res 43:7600–7607
Jin L, Bai R (2002) Mechanisms of lead adsorption on Chitosan/PVA hydrogel beads. Langmuir 18:9765–9770
Bessbousse H, Rhlalou T, Verchere JF, Lebrun L (2009) Novel metal-complexing membrane containing poly(4-vinylpyridine) for removal of Hg(II) from aqueous solution. J Phys Chem B 113:8588–8598
Association of Analytical Chemistry (1990) Official methods of analysis, 15th edn. Washington DC, USA
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2009) Adsorption of copper (II), chromium (III), nickel (II) and lead (II) ions from aqueous solutions by meranti sawdust. J Hazard Mater 170:969–977
Mansur HS, Sadahira CM, Souza AN, Mansur AAP (2008) FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater Sci Eng C 28:539–548
Mansur HS, Oréfice RL, Mansur AAP (2004) Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 45:7193–7202
Andrade GI, Stancioli EFB, Mansur AAP, Vasconcelos WL, Mansur HS (2008) Small-angle X ray scattering and FTIR characterization of nanostructured poly(vinyl alcohol)/silicate hybrids for immunoassay applications. J Mater Sci 43:450–463
Alkan M, Demirbas O, Dogan M (2007) Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Micropor Mesopor Mat 101:388–396
Xiao S, Ma H, Shen M, Wang S, Huang Q, Shi X (2011) Excellent copper(II) removal using zero-valent iron nanoparticle-immobilized hybrid electrospun polymer nanofibrous mats. Colloids Surf A 381:48–54
Li X, Li Y, Zhang S, Ye Z (2012) Preparation and characterization of new foam adsorbents of poly(vinyl alcohol)/chitosan composites and their removal for dye and heavy metal from aqueous solution. Chem Eng J 183(88–97):26
Senthilkumaar S, Varadarajan PR, Porkodi K, Subbhuraam CV (2005) Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J Colloid Interf Sci 284:78–82
Costodes VCT, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater 105:121–142
Keshtkar AR, Irani M, Moosavian MA (2013) Comparative study on PVA/silica membrane functionalized with mercapto and amine groups for adsorption of Cu(II) from aqueous solutions. J Taiwan Inst Chem Eng 44:279–286
Hameed BH, Mahmoud DK, Ahmad AL (2008) Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. J Hazard Mater 158:65–72
Aklil A, Mouflih M, Sebti S (2004) Removal of heavy metal ions from water by using calcined phosphate as a new adsorbent. J Hazard Mater 112:183–190
Ho YS, Chiang TH, Hsueh YM (2005) Removal of basic dye from aqueous solution using tree fern as a biosorbent. Process Biochem 40:119–124
Almeida CAP, Debacher NA, Downs AJ, Cottet L, Mello CAD (2009) Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J Colloid Interf Sci 332:46–53
Repo E, Warchol JK, Kurniawan TA, Sillanpää MET (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chem Eng J 161:73–82
Poots VJP, McKay G, Healy JJ (1978) Removal of basic dye from effluent using wood as an adsorbent. Water Pollut Contr Fed 50:926–939
Igwe JC, Abia AA (2007) Adsorption kinetics and intraparticulate diffusivities for bioremediation of Co(II), Fe(II) and Cu(II) ions from waste water using modified and unmodified maize cob. Int J Phys Sci 2:119–127
Ijagbemi CO, Baek MH, Kim DS (2009) Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 166:538–546
Cabal B, Ania CO, Parra JB, Pis JJ (2009) Kinetics of naphthalene adsorption on an activated carbon: comparison between aqueous and organic media. Chemosphere 76:433–438
Sivaraj R, Namasivayam C, Kadirvelu K (2001) Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions. Waste Manage 21:105–110
McKay G, Otterburn MS, Aga JA (1987) Intraparticle diffusion process occurring during adsorption of dyestuffs. Water Air Soil Poll 36:381–390
Ofomaja AE (2010) Intraparticle diffusion process for lead (II) biosorption onto mansonia wood sawdust. Bioresour Technol 101:5868–5876
Urano K, Tachikawa H (1991) Process-development for removal and recovery of phosphorus from waste-water by a new adsorbent 2: adsorption rates and breakthrough curves. Ind Eng Chem Res 30:1897–1899
Acknowledgments
This work was partially financially supported by the Special Computer Science Project, Department of Fundamental Science and Physical Education, Faculty of Science at Siracha, Kasetsart University. We acknowledge the Faculty of Agriculture and Natural Resource, Rajamangala University of Technology Tawan-Ok, Bangpra Campus, for providing AAS experimental.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamnongkan, T., Wattanakornsiri, A., Wachirawongsakorn, P. et al. Effects of crosslinking degree of poly(vinyl alcohol) hydrogel in aqueous solution: kinetics and mechanism of copper(II) adsorption. Polym. Bull. 71, 1081–1100 (2014). https://doi.org/10.1007/s00289-014-1112-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1112-7