Abstract
The focus of the development of UV-crosslinkable self-adhesive medical products is on one hand directed toward customer-oriented requirements such as tack, adhesion, cohesion, biocompatibility, and permeability for water vapor or air. The customer wants highly tolerable, breathable products, which are also characterized by very good skin and optimal release. On the other hand, the economic targets of medical products manufactures must be considered. Development in the area of UV-crosslinkable acrylic pressure-sensitive adhesives (PSA) for medical application describes the variety of acrylic composition, residue monomers content, quality control of peel adhesion level and repeating during the time, biocompatibility of the acrylic self-adhesive layers and their practical medical application. The new class of unsaturable copolymerizable photoinitiator, such as 4-acryloyloxy benzophenone was used for the synthesis of photoreactive UV-crosslinkable solvent-borne acrylic PSA. The properties of acrylic PSA were determined as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pressure-sensitive adhesives (PSA) are being used for a wide range of self-adhesive materials represented by adhesive double-sided, one-sided or carrier-free tapes, adhesive labels, protective foils as technical products and medical pads, hydrogels and biomedical electrodes as typically medical articles [1–3].
Under diverse PSA based on suitable kinds of polymers, such as acrylic, rubber, silicones, polyurethanes, polyesters, polyether, and ethylene-vinyl acetate copolymers (EVA) even acrylic offer promising advantages in comparison to other groups of polymers. PSA acrylics can be applied in forms as a solvent-borne, as a water-borne (dispersions) and as a solvent-free system. Solvent-borne acrylic PSAs offer several advantages, such as excellent aging characteristics and resistance to elevated temperatures and plasticizers, exceptional optical clarity due to the polymer compatibility and non-yellowing. They also have the highest balance of adhesion and cohesion and an excellent water resistance [4].
The performance requirements of medical PSAs based on acrylics are demanding as their ability to adhere well to varying skin types (both dry and moist), they must be removable without leaving adhesive residue or causing skin damage, and should not irritate the skin. Ideally, medical PSA adheres strongly to the skin but can be easily removed with little or no trauma (adhesion properties) and without adhesive residues (cohesion properties). Aggressive PSA may cause skin irritation as well as pain. In the case of long-term wearing of the PSA on the skin, low shear strength values make a patch or bandage esthetically unacceptable, as the adhesive oozes leave adhesive residues on the outside edges of the backing layer. Moreover, when detached, the patch can leave visible residues. Thus, highly critical PSA adhesive properties are peel adhesion and shear strength [5, 6].
The skin, which is the largest organ of the body, averaging 3,000 square inches and 7 pounds in an adult, presents a substantial challenge, as a surface to which medical adhesives must stick. It is a very complex organ consisting of two layers: an inner layer (dermis) and an outer layer (epidermis). Collagen and elastin, the two major proteins of the dermis, form a matrix that supports the epidermis. As the interface between the body and the outside world, the skin provides protection against microbial invasion, controls perspiration for temperature regulation, limits loss of moisture through transpiration, and transmits sensory information [7].
Skin is highly variable with gender, age, ethnicity, location on the body, and ambient conditions. It is also a structurally weak surface. The top-most layer, called the stratum corneum, is made up of cells that have migrated from the base of the epidermis and are in the process of being sloughed off as the skin renews and replenishes itself. The body sheds roughly 10 million cells per day, or about 10,000 cells per waking minute. Skin turns over completely in about 20–30 days, so medical adhesive devices must stick to a layer that is going to be shed [8]. Skin is also a very rough surface, with hair, folds, creases, wrinkles, and pores for sweat and oil glands. Moreover, the surface energy of skin is low, and adhesion is further compromised by contamination with water, oils, salts, and loose debris. As a consequence of these characteristics, which limit surface contact and cause failure of the adhesive bond, mostly in the stratum corneum, there is an upper limit to the degree of adhesion that can be achieved [9] (Table 1).
Three criteria, such as the type of raw material, the available technologies, and the application, establish the basis for trends in the development of adhesives for medical products. The use of highly tolerable substances with minimal allergenic potential is the primary factor with regard to raw materials. The choice is further limited by other external influences [10].
PSAs have been utilized in a range of medical applications, from low-tech pressure-sensitive tapes to high-tech implant adhesives. Typical medical applications include wound coverings and closures, surgical drapes, ostomy mounts, electrocardiograph electrode mounts, electrosurgical grounding pads, and transdermal drug-delivery (TDD) systems. Medical-grade PSAs widely used in wound dressing and in the development of transdermal patches must be biologically inert, non-irritating, and non-sensitizing at the skin level, and should confer good adhesive properties to the final product.
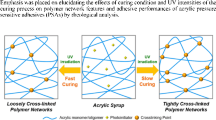
UV-crosslinkable acrylic PSA are becoming increasingly important due to the environmental hazards and medical applications associated with conventional crosslinkable solvent-borne PSAs and the performance shortcomings of PSAs based on aqueous systems. The economics of UV-crosslinking coating technology are also superior to those of typical solvent-borne or water-borne coatings. UV-crosslinkable solvent-borne acrylic PSA systems are available without stabilizers and other additives. So far, the major healthcare applications of that acrylic PSA have been in plaster, medical tapes, self-adhesive bandages, and TDD systems. The major disadvantage of suitable acrylic PSAs in skin-contact applications is the high peel force required to remove them, reflecting their low cohesive strength and hence aggressive nature. The best approach for improving the cohesive strength of these PSAs is to incorporate into polymer chain of special crosslinking agent in form of unsaturated photoinitiators (Fig. 1) [11].
Photoinitiators suitable for polymerization should have good solubility, should react completely in the polymerization process, should be high temperature resistant and should not form photolytic fragments which tend to migrate with a strong specific odor after the UV radiation [12].
Experimental
The following experiments were performed to synthesize of photoreactive UV-crosslinkable solvent-borne acrylic PSA and investigate the influence of a relatively new class of unsaturated copolymerizable photoinitiators representing for 4-acryloyloxy benzophenone (ABP) (Fig. 2) on significant properties of the synthesized acrylic PSA such as tack, peel adhesion, and shear strength after UV exposure.
The acrylic PSA medical grade were synthesized in ethyl acetate at boiling point of solvent during radical polymerization of monomers mixture containing between 64.2 and 64.9 wt% 2-ethylhexyl acrylate (2-EHA), 30% ethyl acrylate (EA), 5% acrylic acid (AA), and between 0.1 and 0.8 wt% unsaturated photoinitiator ABP in the presence of 0.1 wt% radical starter 2,2′-azo-bis-diisobutyronitrile (AIBN). The concentration of monomers blended with AIBN into ethyl acetate before the polymerization process (reactor charge) was 50 wt%, the rest of monomers mixture was added into polymerization reactor during 1 h at conducted post-reaction time of 5 h (minimizing of free monomers). The monomers: 2-EHA, EA, and acrylic acid (AA) were purchased from BASF (Germany). Thermal starter AIBN and copolymerizable photoinitiator ABP are available from ChemCycle (Germany).
The viscosity of investigated solvent-borne acrylic PSA was determined at 23 °C with a Rheomat RM 189 from Rheometric Scientific, with spindle No 3.
The amount of solid materials was found according to DIN EN 12092. The residual of monomers were measured with gas chromatograph Unicam 610, J&W DB-1 column, FID detector, and integrator Unicam 4815.
The number average molecular weight, weight average molecular weight, and molecular weight distribution studies were performed in tetrahydrofuran with a liquid chromatography LaChrom system: RI Detector L-7490 and LaChrom UV Detector L-7400 from Merck-Hitachi, equipped with a PLgel 106 Å column from Hewlett-Packard.
The UV-inducted crosslinking process was performed using an UV light lamp U 350-M-I-DL from IST Company with a UV-A wavelength between 315 and 380 nm. The UV exposure was measured using an integrating radiometer Dynachem™ Model 500, available from Dynachem Corporation, 2631 Michelle Drive, Tustin, CA 92680.
The representative solvent-borne acrylic PSAs, synthesized with different amounts of ABP, were coated at a coating weight of 60 g/m2 solid content onto a smooth layer of polyester film, and after drying for 10 min at ca. 105 °C the adhesives were crosslinked by UV radiation using mentioned UV lamp.
The influence of the ABP photoinitiator on UV-initiated crosslinking of photoreactive synthesized acrylic PSA is usually determined in relation to the photoinitiator concentration, UV-crosslinking time, and UV dose to the significant PSA properties such as tack, peel adhesion, and shear strength. The first three properties were determined by standard Association des Fabricants Europeens de Rubans Auto-Adhesifs (AFERA) procedures. Exact details can be found in AFERA 4015 (tack), AFERA 4001 (peel adhesion), and AFERA 4012 (shear strength). Administrative address: 60 rue Auber-94408 Vitry Sur Seine Cedex, France.
Results and discussion
Viscosity of synthesized acrylic PSA
The viscosity of the solvent-borne acrylic PSA was determined for different concentrations of unsaturated photoinitiator ABP that was incorporated into polymer chain during radical polymerization process of monomers mixture (Fig. 3).
As shown in Fig. 3, for solvent-borne acrylic PSA synthesized with ABP, the increase of the ABP concentration causes in increase of the PSA viscosity. All measured viscosities of acrylic PSA containing between 0.1 and 0.8 wt% ABP are acceptable for coating process on professional coating machine.
Molecular weight and molecular weight distribution of synthesized acrylic PSA
With various concentrations of the acryloyloxy-photoinitiator ABP incorporated into acrylic polymer chain, the molecular weight and molecular weight distribution of solvent-borne acrylic PSA are listed in Table 2.
As it can be stated from tested acrylic solvent-borne PSA containing ABP, ABP shows generally positive influence on all the evaluated molecular weights and molecular weight distribution known as polydispersity (P d). The incorporation of 0.8 wt% ABP improved the weight average molecular weight (\( \bar{M}_{\rm{w}} \)) by about 3%, increased the number average molecular weight (M n) by about 3.1%, and simultaneously decreased the polydispersity (P d) by about 6.4%.
Determination of free monomers in synthesized acrylic PSA
The free unreacted acrylate monomers in synthesized solvent-borne acrylic PSA were determined using headspace GC chromatography. The results of this measurement were presented in Table 3.
The very low level of free acrylate monomers into photoreactive UV-crosslinkable synthesized solvent-borne acrylic PSA predestinates clear this kind of acrylic PSA for medical applications.
Properties of photoreactive solvent-borne acrylic PSA after UV-crosslinking
The investigations with different exposure times of UV radiation and at diverse UV dose were carried out with solvent-borne acrylic PSA containing unsaturated photoinitiator ABP in a concentration ranging from 0.1 to 0.8 wt%. The UV-crosslinking effect of the selected amounts of ABP on following properties of acrylic PSA, such as tack, peel adhesion, and shear strength, depending on crosslinking time, was illustrated in Figs. 4, 5, and 6.
Dependencies shown in Figs. 4 and 5 point out that the UV exposure time influences tack and peel adhesion of acrylic PSA containing unsaturated photoinitiator ABP after UV radiation crosslinking. A reduction of tack was observed with increasing ABP concentration and with longering of the UV exposure time. After about 1 min of UV-crosslinking time, the tack values reach a stable level. In the case of peel adhesion, maxima for selected ABP concentrations and fixed UV-curing times were observed. Longer curing times are in line with smaller ABP amounts. For example, for 0.1 wt% ABP, the peel adhesion maximum was observed at 2 min UV exposure. For 0.8 wt% ABP, this maximum was stated at a UV-crosslinking time of 1 min.
When comparing selected concentrations of ABP, the latter brought about a more rapid increase of shear strength at the measured temperatures 20 and 70 °C (Fig. 6), in comparison with other previously investigated unsaturated photoinitiators. Excellent cohesion values, of 120 N at 20 °C and 40 N at 70 °C for 0.8 wt% ABP, were observed after 1 min of UV-crosslinking time. The use of 0.1 wt% ABP yielded maximal shear strength of 100 N at 20 °C and 30 N at 70 °C.
The UV-crosslinking effect of concentration of copolymerizable photoinitiator ABP ranging from 0.1 to 0.8 wt% on tack, peel adhesion, and shear strength at UV doses between 50 and 250 mJ/cm2, for 3 min UV-crosslinking time, is shown in Figs. 7 and 8.
The increase of the UV dose is affected also by the fact that during UV exposure more free radicals arise from the incorporated ABP and after crosslinking the tack consequently decreases. Above 100 mJ/cm2 UV dose, the tack level changes very slowly (Fig. 7). As can be seen in Fig. 8, the UV dose influences another very relevant parameter of UV-crosslinked PSA, such as the peel adhesion. Also, by a long (3 min) UV-crosslinking time, the use of 0.1 or 0.3 wt% ABP as unsaturated photoinitiator ABP with hydrogen abstractor character is preferred. Maximal peel adhesion values were achieved for UV doses between 100 and 200 mJ/cm2.
Summary
It should be noted from the performed investigations that an almost clear relationship exists between the tested tack, peel adhesion, and shear strength values of UV-crosslinked solvent-borne acrylic PSA and the incorporation of acryloyloxy-photoinitiators such as ABP. From the evaluated unsaturated photoinitiator ABP, the best performance was achieved when using 0.3 wt% ABP in all conducted trials by 1 min UV-crosslinking time and UV dose of about 100 mJ/cm2. From the reason that the synthesized solvent-borne acrylic PSA are almost monomer-free, it can be excellent candidate for the practical application their in the medical industry.
References
Lucast DH (2000) Skin tight. Adhes Age 10:36–39
Kummer A (2000) Important properties of synthesized acrylic pressure-sensitive adhesives. Adhes Age 12:40
Webster I (1999) The development of a pressure-sensitive adhesive for trauma-free removal. Int J Adhes Adhes 19:29–34
Cunningham D, Lowery M (2004) Moisture vapor transport channels for the improved attachment of a medical device to the human body. Biomed Microdev 2:149–154
Jin X, Bai YP, Shao L et al (2009) Properties of solvent-borne acrylic pressure-sensitive adhesives synthesized by a simple approach. eXPRESS Polym Lett 12:814–820
Benedek I (2006) Development in pressure-sensitive adhesive products. CRC Press, Taylor & Francis Group, Boca Raton
Minghetti P, Cilurzo F, Tosi L (2003) Design of a new water-soluble pressure sensitive adhesive for patch preparation. AAPS Pharm Sci Tech 4:1–9
Czech Z, Kurzawa R (2007) Acrylic pressure-sensitive adhesive for transdermal drug delivery systems. J Appl Polym Sci 106:2398–2404
Ghosh TK, Pfister WR, Yum SJ (1997) Transdermal and topical drug delivery systems. Interpharm Press Inc., Buffalo Grove
Chalykh AA, Chalykh AE, Novikov MB, Feldstein MM (2002) Pressure-sensitive adhesion in the blends of poly(N-vinyl pyrrolidone) and poly(ethylene glycol) of disparate chain lengths. J Adhes 78:667
Czech Z (1999) Crosslinking of pressure-sensitive adhesives based on acrylic. Technical University of Szczecin, Szczecin
Czech Z, Milker R, Butwin A, Herko E (2008) Removal of organic solvents for the purpose of manufacturing of solvent free pressure-sensitive adhesives. Polish J Chem Technol 1:37–40
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Czech, Z., Kowalczyk, A., Kabatc, J. et al. UV-crosslinkable acrylic pressure-sensitive adhesives for industrial application. Polym. Bull. 69, 71–80 (2012). https://doi.org/10.1007/s00289-012-0725-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-012-0725-y