Abstract
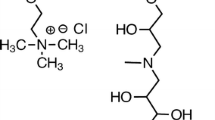
Water-soluble polymer poly[3-methacryloylamine)propyl)trimethyl ammonium chloride, P(ClMPTA) and the copolymer with 4-vinyl pyridine, poly[(3-methacryloylamine)propyl) trimethylammonium chloride-co-4-vinyl pyridine], P(ClMPTA-co-4VP) were synthesized by radical polymerization, at different feed mole ratios ClMPTA:4VP 1:1, 1:2, and 2:1. The copolymer compositions were determined by FT-IR and H-NMR spectroscopy and analyzed by TG-DSC. The liquid-phase polymer-based retention (LPR) technique was used to study the water-soluble polymers’ arsenic removal properties. The solution’s conductivity properties were evaluated at different pH. The copolymers can bind more selectively divalent anionic arsenic species from an aqueous solution (pH 8 ≥ pH 6 > pH 4). Assays for the mol ratio copolymer: As(V) 75:1, 37.5:1, 20:1, 10:1, and 5:1 at arsenic concentrations of 10 and 37.5 ppm were carried out. Apparently, the behavior of the copolymers with the solution’s pH was similar to pure cationic homopolymer; however, when the retention capacity was expressed as real mass of quaternary ammonium comonomer, the retention values were enhanced for lowest mol ratio 10:1 and 5:1. The retention capacity of exchanger with quaternary ammonium group was improved in presence of a weak base 4-vinyl pyridine comonomer, differently to the behavior showed by those copolymers of ClMPTA with acrylic acid groups as comonomer.








Similar content being viewed by others

References
Nriagu JJ (ed) (1994) Arsenic in the environment. Part I: cycling and characterization. Wiley, New York
Jang M, Wang H, Choi S (2007) Chemosphere 66:8
Bissen M, Frimmel F (2003) Acta Hydrochim Hydrobiol 31(1):9
Sarkar S, Blaney L, Gupta A, Ghosh D, Sengupta A (2008) Environ Sci Technol 42(12):4268
Luong J, Majid E, Male K (2007) Open Anal Chem J 1:7
Tournassat C, Charlet L, Bosbach D, Manceau A (2002) Environ Sci Technol 36:493
Dixit S, Hering J (2003) Environ Sci Technol 37:4182
Manning B, Fendorf S, Bostick B, Suarez D (2002) Environ Sci Technol 36:976
Martin T, Kempton J (2000) Environ Sci Technol 34:3229
Pookrod P, Haller K, Scamehorn J (2004) Sep Sci Technol 39(4):811
Wakui Y, Persulessy A, Ikeda TJ, Ebina T, Onodera Y, Suzuki T (2005) Anal Sci 21:433
De Marco M, Sengupta A, Greenleaf J (2003) Water Res 37:164
Cumbal L, Sengupta A (2005) Environ Sci Technol 39:6508
Rivas BL, del Aguirre MC (2007) J Appl Polym Sci. 106:1889
Rivas BL, Pereira E, Moreno I (2003) Progr Polym Sci 28:173
Wandrey C, Hernandez-Barajas J, Hunkeler D (1999) Adv Polym Sci 145:123
Rivas BL, del Aguirre MC, Pereira E (2006) J Appl Polym Sci 102:2677
Rivas BL, del Aguirre MC, Pereira E, Moutet JC, Saint Aman E (2007) Polym Eng Sci 47:1256
Rivas BL, del Aguirre MC, Pereira E (2007) J Appl Polym Sci 106:89
Pu H (2003) Polym Int 52:1540
Barron R, Fritz J (1984) J Chromatography 284:13
Tsuchida E, Abe K (1982) Adv Polym Sci 45:1
Bekturov E, Bimendina L (1981) Adv Polym Sci 41:99
Roiter Y, Minko S (2005) J Am Chem Soc 127:15688
Puterman M, Koenig JL, Lando JBJ (1979) Macromol Sci Phys B16:89
Dobrynin AV, Rubinstein M, Obukhov SP (1996) Macromolecules 29:2974
Roiter Y, Jaeger W, Minko S (2006) Polymer 47:2493
Acknowledgements
The authors thank to FONDECYT (Grant No 1070542), PIA (Grant ACT 130), and “Centro de Investigación de Polímeros Avanzados”, CIPA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivas, B.L., del Aguirre, M.C. Water-soluble copolymers in conjunction with ultrafiltration membranes to remove arsenate ions. Polym. Bull. 67, 441–453 (2011). https://doi.org/10.1007/s00289-010-0393-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-010-0393-8