Abstract
This study is interested in the removal of Pb(II), Cd(II), Co(II), Zn(II), and Sr(II) onto polyacrylic acid acrylonitrile talc P(AA-AN)-talc nanocomposite. P(AA-AN)-talc was fabricated using γ-irradiation-initiated polymerization at 50 kGy. Different analytical tools were used to investigate the functional groups, morphology, particle size, and structure of this composite. The ability of P(AA-AN)-talc to capture (Pb2+, Cd2+, Co2+, Zn2+, and Sr2+) as multi-component aqueous solutions was performed by a batch method. Saturation capacity and the effect of (agitating time, pH, initial metal concentrations, and temperature) were investigated. The distribution coefficients at different pHs have order: Pb2+ > Cd2+ > Co2+ > Zn2+ > Sr2+. The saturation capacity decreases by increasing heating temperatures. Reaction kinetic obeys the pseudo-second-order model. Sorption isotherms are more relevant to a Langmuir isotherm, and the monolayer sorption capacity is closed to saturation capacity. Thermodynamic parameters (∆G˚, ∆H˚, and ∆S˚) were endothermic and spontaneous. P(AA-AN)-talc is used for loading and recovery of studied cations in the column system. The study confirms that P(AA-AN)-talc is a promised composite for the sorption of the studied ions from aqueous solutions and should be considered as potential material for decontaminating these ions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radioactive waste is obtained through a wide scale of operations in research facilities, recycling plants, power plants, diagnostic medicine, and isotope manufacturing (Nilchi et al. 2007; Hamed et al. 2016b). The stream of radioactive waste from nuclear facilities includes low, intermediate, and highly radioactive wastes and also includes emitting isotopes. Furthermore, these streams could contain different hazardous elements from decontamination procedures. These wastes must be treated to decrease dangerous element concentrations to levels suitable for discharge into the environment (Nilchi et al. 2007). Recently, the contaminants captured from wastewater have become a major challenge, as their significance is extending with increasing industrial activities (Hamoud et al. 2014). Various technologies can be used to remove pollutants as, for example, chemical precipitation (Ahn et al. 2020), physical treatment like solvent extraction (Brockmeyer et al. 2015; Hurtado-Bermúdez et al. 2018), adsorption (Abass et al. 2021a, b), biosorption (Gupta et al. 2019), membrane (Mehta et al. 2020), and ion exchange (El-Dessouky et al. 2018). Because of the high toxicity of Pb(II) and their widespread presence in the area, lead ions have a particular interest. It is an industrial contaminant, which arrives in the ecosystem through the soil, air, and/or water (Arbabi et al. 2015). Cadmium is common for electroplating, nuclear reactors, and certain industrial paints (Aglan et al. 2019). Cd(II) has several isotopes along with 109Cd; its half-life is 1.27 years and is used as rods for controlling and shielding the absorption of neutrons in reactors with supplementary components (Aglan et al. 2019). Co(II) is used in nuclear, medical, enamel, semiconductor industries, and electricity galvanizing. Co(II) can cause adversative effects on health like asthma, heart damage, and destroying the thyroid gland and liver (Kulkarni 2016). Zinc is obtained through effluents discharged from industries, like pigments, electroplating, battery manufacturing, metallurgy, and municipal wastewater management plants. Zn(II) is a toxic ion and can cause damage to human life by bioaccumulating in the food chain (Zhang et al. 2017). Strontium radionuclide is formed by 89Sr (t0.5 = 51d) and 90Sr (t0.5 = 29y) and is produced not only from nuclear plants as fission radionuclides but also from nuclear fuel rods in the pre-treatment phase (Ali et al. 2020). The retention of Cd(II), Co(II), Pb(II), Zn(II), and Sr(II) from liquid and radioactive waste solutions has been achieved by many researchers (Wang et al. 2010; Dubey et al. 2014; Kang et al. 2016; Zhang et al. 2017; Aglan et al. 2019; Pyrzynska 2019).
Talc ore is a naturally occurring, low-cost, and small particle size material. Talc ore has a platy structure that consists of three layers, including the Mg-OH layer sandwiched among two (SiO3)2− layers. Adjacent layers are linked by van der Waals forces. Talc powder surface has a huge number of Si–O-Si, Mg-O, OH, and O-Si–O bonds which will coordinate with transition elements in H2O or soil to capture them on the talc powder surface (Thi Huong et al. 2020). Talc is commonly used as a filler, coating, and dusting agent in paints, plastics, lubricants, pharmaceuticals, papers, cosmetics, and ceramics manufacture. There are few studies on the adsorption characteristics of heavy metal ions in talc (Andrić et al. 2014; Kalantari et al. 2014). Modified talcum powder using 50% HNO3 acid was used for sorption of methylene blue from wastewater (Wenlei et al. 2014). Fe3O4/Talc nanocomposite was fabricated by the coprecipitation-ultrasonication technique and used for the capture of Cr(IV) from aqueous solutions (Thi Huong et al. 2020). Fe3O4/talc nanocomposite was used for the removal of Cu(II), Ni(II), and Pb(II) ions from aqueous solutions (Kalantari et al. 2014).
Polymeric resins have some advantages like high capacity, non-toxic, high chemical stability, and low production cost, but they have some disadvantages such as low selectivity toward hazardous ions, ability to swilling, low radiation, and thermal stability. The sorption efficiency of the talc can be improved by adding polymeric resins. The impregnation of talc ore to organic polymer is more useful due to creating new materials having higher sorption capacity, fast adsorption rate, and selectivity for target elements, as well as reducing some disadvantages present in polymeric resins. The novelty of this study is the impregnation of talc inside polymeric resin layers using the gamma radiation technique as a new nanocomposite with high sorption performance for some metal ions from aqueous solutions, in addition to the need to develop and use new economic materials that have specifications suitable for working in radioactive waste treatment conditions.
In the present work, the fabrication and characterization of P(AA-AN)-talc nanocomposite using gamma radiation at 50 kGy were achieved. The sorption behavior of Cd(II), Co(II), Pb(II), Zn(II), and Sr(II) onto P(AA-AN)-talc nanocomposite is discussed. Chromatographic separations of binary and multi-component systems were applied on P(AA-AN)-talc nanocomposite.
Experimental
Materials
All salts and reagents in this work have an analytical grade and are used without further purification. CoCl2.6H2O, SrCl2, PbNO3, ZnCl2, CdCl2, acrylic acid (AA), and acrylonitrile (AN) from Alpha Chemika, India; HCl and HNO3 from Merck, Germany; and sodium hydroxide from El-Nasr Co., Egypt. Double distilled water (DDW) was used for all experiments and nanocomposite fabrication.
Gamma cell
Co60 γ-cell source of class MC-20 (Russia) was operated as radiation of polymerization process at the Cyclotron factory, Egypt. The rate of the dose was ~ 473.35 Gy/h.
Fabrication of P(AA-AN)-talc
At 303 ± 2 K and constant agitation, 10% (AA and AN) solutions were added dropwise to 10% talc solution, and 0.1% N, N-methylene-bisacrylamide (cross-linker) was distributed for the production of P(AA-AN)-talc with volumetric ratios (AA:AN: talc) equal unity. The solution remained agitated for 2 h to overcome complete homogeneity then subject to γ-irradiation at 50 kGy over a time of about 105 h. After radiation, the hydrogel was cut into small fragments, soaked in acetone to eliminate unreacted monomers, then washed with DDW, and dried at 333 ± 2 K. The solid was ground to powder and converted into the H+ form by mixing for 1 day with 0.1 M HNO3. The solid product was decanted and rinsed with DDW to eliminate the excess acid and dried at 333 ± 2 K.
Instruments
The studied material was analyzed by Fourier transform infrared spectroscopy (FT-IR) (Alpha II Bruker, Germany) at 4000–400 cm−1. X-ray diffraction (XRD) was done (Shimadzu XD-D1, Japan). Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were done by a Shimadzu DTG-60 H. The surface morphology of the solid was recorded by scanning electron microscope (SEM) model Philips XL 30. The percent of elemental composition was detected by energy-dispersive X-ray (EDX) analysis. Transmission electron microscopy (TEM) and selected area electron diffraction (SAED) pictures were performed using JEM2100, Jeol. s.b, Japan. Atomic absorption spectrophotometer (Buck Scientific, VGP 210) was used to measure the concentrations of Cd(II), Co(II), Pb(II), Zn(II), and Sr(II).
Chemical stability
The stability of P(AA-AN)-talc to different solvents was achieved by mixing 0.05 g of solid and 50 mL of H2O, HNO3, HCl, and NaOH in concentrations [1–6 M] in the desired solution with intermittent agitating for about three days at 298 ± 1 K. The filtrates were dried using IR lamps to dry, and then, the residue was tested gravimetrically by weight difference by a sensitive analytical balance(Abou-Mesalam et al. 2018).
Point zero charge (PZC) determination
PZC for P(AA-AN)-talc was investigated by using a 10-mL bottle, and 0.1 g of solid was added to 10 mL of desired pH solutions. The pH was adjusted by 0.1 M (HCl and NH3) to find the different pHs of (2–12). The pH of the supernatant in each tube was represented as pHi. The samples were agitating for 24 h using a rotary agitator at 200 rpm. After settling, the pH of the supernatant in each tube was measured and represented as pHf. The PZC was determined from the plot of ΔpH versus pHi.
Adsorption experiments
Preliminary study
The experiment was done by shaking 0.05 g of two samples (talc and P(AA-AN)-talc) with 5 mL of Pb(II), Cd(II), Co(II), Zn(II), and Sr(II) (100 mg/L) with V/m = 100 mL/g in a shaker thermostat (Kottermann D-1362, Germany) at 300 ± 1 K. Sorbent and the sorbate solution were agitated in a shaker thermostat at 180 rmp and after sorption (24 h), and the samples were separated from the solution by centrifuge. Ion concentrations in the test solutions were detected before and after sorption by AAS. The uptake percentage is calculated by Eq. (1) (Khataee et al. 2013; Hassani et al. 2014; Hamed et al. 2019):
where Ci and Cf are the initial and final concentrations of sorbed metal ions in solution, respectively, and the data are tabulated in Table 1. These results reveal that the percent uptake was increased from about 68.2 to 96.9% for Pb(II), from about 61.8 to 91.1% for Cd(II), from about 42.2 to 48.9% for Co(II), from about 21.5 to 29.5% for Zn(II), and from about 8.2 to 11.8% for Sr(II); these data confirm that a great enhancement was noticed in the sorption of Pb(II), Cd(II), Co(II), Zn(II), and Sr(II) onto P(AA-AN)-talc, and this modified nanocomposite was used for further experimental work.
Batch sorption studies of the Pb(II), Cd(II), Co(II), Zn(II), and Sr(II) onto P(AA-AN)-talc in H+ form. The variation in sorption parameters such as pH (1–5), contact time (5–240 min), metal ion concentrations (50–600 mg/L), and temperatures (300, 313, and 333 K) is patterned to get the optimum condition for the sorption process.
The separation factor may be considered as the relative tendency of different ions to be adsorbed in an exchanger from solutions. It is used as a measure of the possibility of chromatographic separation and is also expressed as the ratio of the distribution coefficients of the ions to be separated. The distribution coefficients (Kd) and separation factors \((\alpha^{a}_{b} )\) as a function of pH are calculated with the Eqs. (2) and (3) (Metwally et al. 2019):
where Ci and Cf are the initial and final concentrations of Cd(II), Co(II), Pb(II), Zn(II), and Sr(II) in solution, respectively. V is the volume of solution (mL) taken in (5 mL), and m is the mass of the sorbent (g) taken in (0.05), and a and b are two challenging types in a system.
Saturation capacity
Repeated equilibration of 200 mg/L studied cations with the P(AA-AN)-talc in V/m = 200 mL/g was carried out for the saturation capacity determination at different heating temperatures (323–673 K). The mixtures were agitated in an agitator at 300 ± 1 K for 2 h. After equilibrium, the solution was separated, and the concentration of the metal ions was measured. This procedure was repeated many times with fresh solutions until the nanocomposite was saturated with metal ions. The saturation capacity q (mg/g) was calculated from Eq. (4) (Hassan et al. 2021; Ibrahim et al. 2021):
where Ce is the equilibrium concentration of Cd(II), Co(II), Pb(II), Zn(II), and Sr(II); V is the volume of solution (L) taken in (0.1 L); and m is the mass of P(AA-AN)-talc (g) taken in (0.05 g).
Column separation
Chromatographic breakthrough investigations were directed as follows: 0.5 g of solid was packed in a plastic column (0.3 cm radius and 5 cm heights) to give a bed height of 1.1 cm3 volume. At a rated flow of 4 drops/min, 600 mL of 100 mg/L desired metal ions at pH = 4 was passed through the column beds. A breakthrough capacity (BTC) values were computed by the formula (Abass et al. 2021a):
where V50% is a volume for effluent at 50% breakthrough (L).
Double distilled water and different HNO3 concentrations (0.01, 0.05, 0.1, 0.2, and 0.5 M) were used to elute the loaded ions from the P(AA-AN)-talc surface at the flow rate of four drops/min, and the eluent was collected from every 20 min, and the concentrations were continuously measured.
Results and discussion
FTIR
FTIR spectrum of P(AA-AN)-talc is shown in Fig. 1(A). This figure shows that bands at 3739 and 3568 cm−1 are due to H2O and OH absorbed on P(AA-AN)-talc (stretching vibration) (Abass et al. 2022b). A strong band at 2229 cm−1 is due to the C≡N bond of acrylonitrile (stretching vibration) (Lee et al. 2012a). Two bands at 1574 and 1511 cm−1 are due to N–O (stretching vibration) (Dawood and Li 2014). The band at 1395 cm−1 is due to O–H of carboxylic acid (bending vibration) (Lee et al. 2012b). The band at 1212 cm−1 is due to symmetric C-N (bending vibration) (Dawood and Li 2014). The band at 951 cm−1 is attributed to Si–O-H deformation vibration (Borai et al. 2015) or Si-CH2 (Mirzayev et al. 2021). The band at 722 cm−1 may be attributed to the Si–O-M (where M = Mg, Ca, and/or Al) (Nabi et al. 2011; Abass et al. 2022a). The band at 478 cm−1 may be attributed to the Si–H (Mirzayev et al. 2021). The three later bands deep-rooted the in-situ precipitation of talc (Mg, Al, and Si) in the net of a polymeric compound as seen later in EDX analysis.
XRD analysis
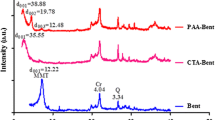
XRD analysis of P(AA-AN)-talc nanocomposite is shown in Fig. 1(B). This figure indicated that P(AA-AN)-talc has a crystalline structure and exhibits many sharp peaks centered at 6.19˚, 18.64˚, 19.64˚, 20.9˚, 21.86˚, 24.94˚, 26.88˚, 28.36˚, 31.32˚, 34.61˚, 35.17˚, 36.7˚, 39.83˚, 44.67˚, 50.08˚, and 60.0˚ related to Miller index indications (020, 002, 003, 11–1, 111, 11–2, 004, 023, 113, 005, 131, 201, 132, 133, 116, 045, and 060), respectively, with JCPDS number 00–901-0163 confirming their crystalline nature with a monoclinic system, and this result was parallel to the XRD of chlorite (Zanazzi et al. 2007), Fe3O4/Talc nanocomposite (Thi Huong et al. 2020), and talcum powder (Wenlei et al. 2014).
Thermal analysis
The thermal analysis (TGA and DTA) of P(AA-AN)-talc nanocomposite (Fig. 2), showing that the weight loss process occurred via three steps. From 367 to 583 K due to the lessening of all moisture and free H2O of P(AA-AN)-talc (Hamoud et al. 2014), the lost weight is 7.5%. From 583 to 733 K due to subtraction of H2O of crystallization (Abass et al. 2021b) and complete decay of the organic part of P(AA-AN)-talc (El-Aryan et al. 2015), the lost weight is 34.2%. The third stage of weight loss (15.7%), which occurred from 733 to 1073 K caused by heating in N2 gas and the cycling reaction with C ≡ N bonds converted to C = N bonds (El-Aryan et al. 2015). DTA shows 2 peaks at 379 K and 664 K (endothermic), due to the subtraction of all surface and matrix-bound H2O from the polymeric compound and dehydration of carboxylic acid and decarboxylation of acrylic acid, respectively. Two exothermic peaks at 458 K and 592 K correspond to comprehensive decomposition of the organic molecule. From the data of TGA (Fig. 2), the losses weight is continued up to 1073 K. The full losses weight for P(AA-AN)-talc with the heating temperature is 57.7%.
SEM
The performance of the particulate composite is detected by particle dispersion inside the matrix; a smooth particle distribution contributes to uniform powder properties, as it is shown in Fig. 3(A). Proper talc dispersion is noticed at diverse points of the sample cross sections with no presence of agglomeration. Also, a favored orientation of talc is appreciated for the composite, resulting from their plate-like structure (Andrić et al. 2014).
EDX
Figure 3(B) shows clearly distinct phases of Al, Mg, Si, O, and C, and the resulting relative percentages of different metals at the points are C = 55.3%, O = 20.2%, Si = 7.16%, Al = 6.3%, Mg = 0.29%, and Ca = 1.2. These results confirmed the impregnation of talc in polymeric resin layers as seen in the FTIR analysis mentioned earlier.
TEM
TEM images of P(AA-AN)-talc (Fig. 4(A)) were used to estimate small particle size and were extended from 3.1 to 6.34 nm. Also, P(AA-AN)-talc shows a spherical morphology. Also, the TEM image shows a good distribution of talc inside the polymeric resin. The selected area electron diffraction (SAED) pattern (Fig. 4(B)) shows the central, intense, and direct beam such as a pattern, with a sharply focused spot, confirming that P(AA-AN)-talc is at least partly crystalline (Carter et al. 1996). And these results confirm data calculated from XRD as mentioned earlier. The average particle size of the new material is 4.99 nm.
Stability against chemicals
The chemical stability of P(AA-AN)-talc towards different solvents (H2O, HNO3, HCl, and NaOH) was performed and the results are tabulated in Table 2 and indicated that the prepared P(AA-AN)-talc is stable in H2O and HCl; also, the investigated sample is stable in HNO3 and NaOH up to 3 M; meanwhile, it starts to dissolve at 4 M and greatly dissolves at 6 M (Abou-Mesalam et al. 2018). Also, these results reveal that P(AA-AN)-talc is stable according to sequence order: HCl ˃ HNO3 ˃ NaOH.
Point zero charge
Point zero charge (PZC) for P(AA-AN)-talc was determined by using plots of ΔpH vs pHi (Fig. 5). From this Figure, it is clear that at acidic medium (pH < 7) the ∆pH increases with increasing pHi then the ΔpH decreased at alkaline medium (pH > 7), and PZC was determined at pH value (2.7); this value revealed that the surface charge of P(AA-AN)-talc becomes neutral at this value, and above this value, it becomes negatively charged which leads to improve cationic species, whereas the surface of P(AA-AN)-talc is positively charged below this pH value which leads to improve anionic species.
Adsorption investigations
Distribution coefficients (Kd)
Figure 6(A) shows the Kd difference of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ (100 mg/L) on P(AA-AN)-talc in H+ form, as a function of pH. The Kd improves with the increase in pH. At lower pH lower than three, Kd of the studied cations was inhibited; this is attributed to the existence of excess protons competing with Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ in the solution and preferably occupying the binding sites existing in P(AA-AN)-talc (Metwally et al. 2019). At pH ≥ 3, Kd continuously increases with the increase in pH due to the decline in proton competition and optimum uptake achieved at pH 4 as well as uptake was slightly decreased at pH higher than 4, due to the studied cations starting to precipitate, and all experimental work was done at pH 4. Distribution coefficients (Kd) and separation factors \((\alpha^{a}_{b} )\) for the mentioned cations in different pHs (1–5) are computed and presented in Table 3. The results reveal that Kd values have the sequence: Pb2+ > Cd2+ > Co2+ > Zn2+ > Sr2+ reflecting that the sorption of metal ions was achieved in hydrated ionic radii except Sr2+ applied in unhydrated ionic radii (Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ have ionic radii 1.2, 0.97, 0.63, 0.74, and 1.13 Å, respectively) according to this sequence (Abou-Mesalam et al. 2020). Separation factor \((\alpha^{a}_{b} )\) values were computed and revealed that Pb2+ has very higher \(\alpha^{a}_{b}\) by 284.8, 78.1, 33.3, and 3.5 for Sr2+, Zn2+, Co2+, and Cd2+ at optimum uptake (pH 4); these values indicated that Pb(II) ion can very easily be separated from radioactive and industrial waste solutions, which reflected no selectivity for Sr2+. Non-linear relations between log Kd and pH were observed for studied cations as exposed in Fig. 6(B). The non-ideality of the exchange reaction was clarified from this relation. The difference may be due to the eminence of a mechanism other than ion exchange, such as precipitation and surface sorption (Abou-Mesalam et al. 2018).
Agitating time impact
The influence of shaking time changed from 5 to 240 min on the sorption efficiency of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ (100 mg/L) onto P(AA-AN)-talc was done at pH 4, and the experimental results are given in Fig. 7(A), as a relation between sorption efficiency percent and time. From this figure, it is clear that, as the mixing time increases from 5 to 120 min, the sorption percent of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ increased from about 72.5 to 96.9% for Pb2+, from about 55.1 to 91.1% for Cd2+, from about 23.8 to 48.95% for Co2+, from about 12.8 to 29.5% for Zn2+, and from about 5.2 to 11.8% for Sr 2+. Further, an increase in the mixing time up to 240 min does not affect the removal of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+. This means that the sorption equilibrium has been achieved at 2 h. Therefore, 2 h represents the preferred time to maximize the Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ sorption using the P(AA-AN)-talc, and this time was used for all experiments.
Saturation capacity and thermal stability
The influence of heating temperature on the capacity of studied cations on P(AA-AN)-talc in H+ form is detected at 300 ± 1 K, and the data are presented in Table 4. The prominent reduction in the capacity was observed with rising temperature; it is related to the decay of the organic part of the polymeric material with an increase in temperatures as exposed in TGA and DTA data (Gupta et al. 2015). The capacities of studied cations have sequence order: Pb2+ > Cd2+ > Co2+ > Zn2+ > Sr2+; this order reveals that the sorption process was achieved according to the lessening in the hydrated ionic radii and hydration energy (Gupta et al. 2003; Rahman et al. 2017). The high capacity of the material investigated for Pb2+ and Cd2+ may be due to the higher complexing ability of these ions (Abou-Mesalam et al. 2020).
Kinetic investigation
The pseudo-first-order and pseudo-second-order equations are specified as (Gürses et al. 2014):
where Kf (min−1) and Ks (g mg−1 min−1) are the rates constant of pseudo-first-order and pseudo-second-order, respectively, and qe and qt (mg/g) are the amounts sorbed per gram at equilibrium and time t. Plotting log (qe-qt) and t as shown in Fig. 7(B), the plot shows a linear relationship. From Table 5, it is found that the pseudo-first-order model does not apply to Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ sorption onto P(AA-AN)-talc. When the qe calculated from pseudo-first-order plots was compared with the qe (experimental) considered, one of the main discrepancies was observed (Sheha and El-Zahhar 2008). However, pseudo-second-order is obtained from plotting t/qt against t for Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ sorption as shown in Fig. 7(C), and it is found that the relationship is linear. The data represented in Table 5 reveal that the correction coefficients (R2) are closed to unity which indicates that the sorption procedure follows the pseudo-second-order model; also, the values (qe, Kf, and Ks) confirm that the pseudo-second-order model applies to Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ sorption onto P(AA-AN)-talc. Similar phenomena have been observed for Pb(II) and Cd(II) adsorption on biochars (Park et al. 2013), Sr(II), and Cs(I) sorbed by irradiated saccharomyces cerevisiae (Tan et al. 2017), cobalt(II) sorbed by orange peel waste (Altunkaynak et al. 2021), and adsorption of Zn(II) on natural bentonite (Sen and Gomez 2011).
Mechanism of adsorption
The adsorption mechanism was examined using of intra-particle diffusion model (Karaca et al. 2013; Dakroury et al. 2021).
where C (intercept) and Kid (slope) is the intra-particle diffusion rate constant (mg min−0.5 g−1). The intra-particle diffusion model for the sorption process of the studied metal ions was established through three steps (Karaca et al. 2013; Dakroury et al. 2021). The first stage includes the diffusion of metal ions from the solution to the surface of the P(AA-AN)-talc (from 5 to 15 min). The second stage (from 30 to 90 min) describes the gradual sorption on the surface of the P(AA-AN)-talc, which may be the rate-limiting step. The third stage (from 120 to 240 min) is the equilibrium saturation. The relation between qt and t0.5 is represented in Fig. 7(D), and the three steps of the sorption mechanism are observed in this figure. The intercept which is the thickness of the surface gave information about the contribution of the surface sorption in the rate-determining step. The larger the intercept, the greater its contribution. Table 6 shows the parameters obtained from the second part of the linear plot. The sorption mechanism of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ onto P(AA-AN)-talc was found to be rapid at the initial period of contact time and then to become constant with the increase in contact time. The multi-diffusion step is the main factor in the control of the sorption mechanism, which includes both film and intra-particle diffusion.
Sorption isotherms
The Langmuir equation relates the concentration of a medium above a solid phase surface at a constant temperature to cover the sorbed molecules on the solid phase surface (Hamed et al. 2016a). The Langmuir isotherm model suggests an estimation of the maximum adsorption capacity that occurred by complete monolayer adsorption on the adsorbent surface (Hamed 2014). The Langmuir isotherm can be represented in the next equation (Hamed et al. 2016a):
where Ce is the equilibrium concentration (mg/L), Qmax is monolayer capacity (mg/g), and b is the sorption equilibrium constant related to the energy of sorption. The represented relation between Ce/qe and Ce gives straight lines for both Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ sorbed onto P(AA-AN)-talc nanocomposite as shown in Fig. 8. Table 7 represents the data obtained from the linear form of the Langmuir equation. The correlation coefficients (R2) are 0.992, 0.998, 0.976, 0.991, and 0.996 for Pb2+, Cd2+, Co2+, Zn2+, and Sr2+, respectively. The maximum monolayer capacities (Qmax) obtained from the Langmuir model were (43.8, 37.91, 28.6, 23.6, and 9.09) for Pb2+, Cd2+, Co2+, Zn2+, and Sr2+, respectively.
The separation factor (RL) considers one of the essential characteristics of the Langmuir isotherm model (Hamed et al. 2016a), which can be calculated from the Langmuir constant, b, as the following:
From data present in Table 7, RL values were found to be 0 < RL < 1 reflecting the favorable sorption isotherms of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ (Hamed et al. 2016a).
The Freundlich isotherm model is an empirical formula that accurately represents multi-layer sorption on heterogeneous surfaces (Abass et al. 2021a). A Freundlich equation can be represented in linear form as:
where KF (mg/g) and 1/n are Freundlich constants related to adsorption capacity and adsorption intensity. These constants are calculated from both slope and intercept of the linear plot of log qe versus log Ce, respectively. The sorption results of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ onto P(AA-AN)-talc nanocomposite performed by Freundlich isotherms are shown in Fig. 8. Freundlich isotherm parameters (KF, 1/n, and R2) are represented in Table 7. The values of R2 = 0.959, 0.915, 0.97, 0.974, and 0.993 for Pb2+, Cd2+, Co2+, Zn2+, and Sr2+, respectively, were much lower than the Langmuir isotherm values, reflecting the applicability of Langmuir than Freundlich isotherm.
Comparison of monolayer capacity with different sorbents reported in the literature
The monolayer capacities (Qmax) of P(AA-AN)-talc for the sorption of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ was compared with other sorbents reported in the literature. As represented in Table 8, the monolayer capacities of P(AA-AN)-talc toward the studied cations are higher than the previously reported values which suggested that P(AA-AN)-talc is a promising sorbent to remove Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ from aqueous solutions (Annadurai et al. 2003; Anitha et al. 2015; Ghasemi et al. 2018; Abou-Lilah et al. 2020; Hassan et al. 2020; Herrera et al. 2020; Abass et al. 2021a, 2022c; Kayranli 2021; Alsehli 2021; Abou-Mesalam et al. 2022).
Thermodynamic studies
A linear relation between ln Kd of studied cations on P(AA-AN)-talc and 1000/T along with the Van’t Hoff relation (Abdel-Galil et al. 2016) is exposed in Fig. 9:
where (∆S˚, ∆H˚, R, and T) are the entropy change, enthalpy change, the universal gas constant, and absolute temperature. The Kd of the investigated cations improved with increasing temperature from 300 to 333 K (i.e., the Kd reduced with rising 1000/T) and similar phenomena for adsorption of Remazol Red RB by modified clay (Karaca et al. 2013). This enhancement in adsorption with increasing the temperature is corresponding to the acceleration of some originally slow adsorption phases and the creation of some fresh exchangeable sites on the adsorbent layers (Abdel-Galil et al. 2016). From both slopes and intercepts of linear relation displayed in Fig. 9, ∆H˚ and ∆S˚ are computed and tabulated in Table 9. The positive values of both ∆H˚ and ∆S˚ indicate the endothermic nature and increased randomness of solid solution interface during the adsorption of these cations on P(AA-AN)-talc, respectively (El-Naggar et al. 2010; Abdel-Galil et al. 2016).
The free energy change of adsorption ∆G˚ was computed by the relation:
The negative values of ∆G˚ represented in Table 9 reflect that the sorption process is spontaneous and indicates the better sorption of these ions on P(AA-AN)-talc compared with H+ ion (El-Naggar et al. 2010).
Column investigations
Binary system
Figure 10(A) shows breakthrough curves for Pb2+ and Sr2+ (100 mg/L) onto the P(AA-AN)-talc column. The concentrations of respective ions in the effluent to the feed solution (C/Co) vs. effluent volume, V (L) are plotted on a breakthrough curve. The uptake of Pb2+ and Sr2+ per gram for solid is computed from Fig. 10(A) using Eq. (5) and found to be 46.3 and 25.0 mg/g for Pb2+ and Sr2+, respectively. From these consequences, it is found that selectivity followed order: Pb(II) > > Sr(II). This order proved that the sorption process was taking place for Pb2+ and Sr2+ in hydrated ionic radii, similar to the result in the batch method (Abou-Mesalam et al. 2018). And a BTC is lower than the capacity determined from a batch method, due to the fast dynamic motion of investigated cations. From the data above-mentioned, the conclusions can be expected that P(AA-AN)-talc is suitable for the capture of Pb2+ from Sr2+ at pH = 4.
Multi-component system
The breakthrough curves for Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ (100 mg/L for each) onto the P(AA-AN)-talc column are displayed in Fig. 10(B). A breakthrough capacity is computed as mentioned earlier in the binary system from Fig. 10(B) using Eq. (5) and found to be 37.7, 28.9, 13.2, 9.6, and 7.4 mg/g for Pb2+, Cd2+, Co2+, Zn2+, and Sr2+, respectively. From these data, the selectivity followed order: Pb2+ > Cd2+ > Co2+ > Zn2+ > Sr2+. This order proved that the sorption was achieved in hydrated ionic radii; hence, the breakthrough capacity was lower than that obtained by the batch technique, due to the fast dynamic motion of studied cations. From the data above-mentioned, the conclusion can be supposed that P(AA-AN)-talc is a more suitable sorbent for the uptake of all studied cations from liquid solutions in the pH = 4.
Elution
Elution of binary system
The elution results for Pb2+ and Sr2+ are illustrated in Fig. 11(A). The elution of the investigated ions is studied using DDW and dissimilar HNO3 concentrations (0.01, 0.1, and 0.5 M). Sr(II) ions were released using DDW and 0.01 M HNO3 as eluents. However, the separation of Pb(II) ions was released using 0.01 and 0.1 M HNO3 as eluents. By 0.5 M HNO3, the column packed with P(AA-AN)-talc becomes free from any sorbed metal ions and can be reused again for chromatographic separation.
Elution of multi-component system
The elution data for Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ are displayed in Fig. 11(B). The elution of the studied cations is investigated using DDW and different HNO3 concentrations (0.01, 0.05, 0.1, 0.2, and 0.5 M). Figure 11(B) shows that Sr(II) ions were released using DDW as eluent, Zn(II) ions were released using DDW and 0.01 M HNO3 as eluents, and Co(II) ions were released using 0.01 and 0.05 M HNO3 as eluents. However, Cd(II) and Pb(II) ions were released using 0.05, 0.1, and 0.2 M HNO3 as eluents by 0.5 M HNO3, and the column packed with P(AA-AN)-talc becomes free from any sorbed metal ions and can be recycled again for column separation.
Conclusion
In this work, P(AA-AN)-talc was fabricated, characterized, and employed for batch sorption of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ from multi-component aqueous solutions. P(AA-AN)-talc was synthesized by gamma irradiation-induced preparation at 50 kGy as a new nanocomposite. The distribution coefficients at different pH have selectivity order: Pb2+ > Cd2+ > Co2+ > Zn2+ > Sr2+. The saturation capacity of the studied cations decreased by increasing the heating temperature. The sorption reaction is fast, and the reaction equilibrium is attained after 2 h. Pseudo-second-order kinetic is applicable with the sorption reaction mechanism. The sorption isotherm belongs to the Langmuir model, and the monolayer capacities of Pb2+, Cd2+, Co2+, Zn2+, and Sr2+ are 43.8, 37.9, 28.6, 23.6, and 9.09 mg/g, respectively. Thermodynamic parameters displayed that the ion exchange was endothermic and spontaneous. Column investigation reveals that P(AA-AN)-talc is more suitable for binary and multi-system. Finally, P(AA-AN)-talc can be worked as an efficient sorbent used for the separation of Pb2+ from Sr2+ (binary system) using 0.01 and 0.1 M HNO3. Also, P(AA-AN)-talc can be used as a promising sorbent for the separation of all studied cations from liquid waste utilizing DDW and different concentrations of HNO3 as eluents.
Data availability
Yes.
References
Abass MR, El-Masry EH, Ibrahim AB (2021a) Preparation, characterization, and applications of polyacrylonitrile/ball clay nanocomposite synthesized by gamma radiation. Environ Geochem Health 43:3169–3188. https://doi.org/10.1007/s10653-021-00813-5
Abass MR, Ibrahim AB, Abou-Mesalam MM (2021b) Retention and selectivity behavior of some lanthanides using bentonite dolomite as a natural material. Chemical Papers 75:3751–3759. https://doi.org/10.1007/s11696-021-01621-y
Abass MR, Diab HM, Abou-Mesalam MM (2022a) New improved thermoluminescence magnesium silicate material for clinical dosimetry. Silicon 14:2555–2563. https://doi.org/10.1007/s12633-021-01049-9
Abass MR, Elmasry E, El-Kenany WM (2022b) Gamma irradiation-induced preparation of polyacrylonitrile acrylamide nano-silica for removal of some hazardous metals. J Inorg Organomet Polym Mater 32:536–546. https://doi.org/10.1007/s10904-021-02156-1
Abass MR, Maree RM, Sami NM (2022c) Adsorptive features of cesium and strontium ions on zirconium tin(IV) phosphate nanocomposite from aqueous solutions. Int J Environ Anal Chem 1-20.https://doi.org/10.1080/03067319.2021.2016728
Abdel-Galil EA, Ibrahim AB, Abou-Mesalam MM (2016) Sorption behavior of some lanthanides on polyacrylamide stannic molybdophosphate as organic-inorganic composite. Int J Ind Chem 7:231–240
Abou-Lilah RA, Rizk HE, Elshorbagy MA et al (2020) Efficiency of bentonite in removing cesium, strontium, cobalt and uranium ions from aqueous solution: encapsulation with alginate for column application. Int J Environ Anal Chem 1–24
Abou-Mesalam MM, Abass MR, Abdel-Wahab MA et al (2018) Polymeric composite materials based on silicate: II. sorption and distribution studies of some hazardous metals on irradiated doped polyacrylamide acrylic acid. Desalin Water Treat 109:176–187. https://doi.org/10.5004/dwt.2018.22084
Abou-Mesalam MM, Abass MR, Ibrahim AB, Zakaria ES (2020) Polymeric composite materials based on silicate. III-Capacity and sorption behavior of some hazardous metals on irradiated doped polyacrylamide acrylonitrile. Desalin Water Treat 193:402–413. https://doi.org/10.5004/dwt.2020.25816
Abou-Mesalam MM, Abass MR, Zakaria ES, Hassan AM (2022) Metal doping silicates as inorganic ion exchange materials for environmental remediation. Silicon 1–9. https://doi.org/10.1007/s12633-021-01568-5
Aglan RF, Hamed MM, Saleh HM (2019) Selective and sensitive determination of Cd(II) ions in various samples using a novel modified carbon paste electrode. J Anal Sci Technol 10:1–11
Ahn JM, Kim S, Kim Y-S (2020) Selection of priority management of rivers by assessing heavy metal pollution and ecological risk of surface sediments. Environ Geochem Health 42:1657–1669
Ali MMS, Abdel-Galil EA, Hamed MM (2020) Removal of strontium radionuclides from liquid scintillation waste and environmental water samples. Appl Radiat Isot 166:109357
Alsehli BRM (2021) Monolayer sorption capacity of copper(II), cadmium(II), and lead(II) from aqueous solution onto the surface of activated carbon prepared from Terminalia catappa leaf samples with reference to the calculation of thermodynamic parameters from Langmuir isotherm constant. Desalin Water Treat 223:299–315. https://doi.org/10.5004/dwt.2021.27105
Altunkaynak Y, Canpolat M, Yavuz Ö (2021) Adsorption of cobalt(II) ions from aqueous solution using orange peel waste: equilibrium, kinetic and thermodynamic studies. J Iran Chem Soc 1–12
Andrić L, Terzić A, Aćimović-Pavlović Z et al (2014) Comparative kinetic study of mechanical activation process of mica and talc for industrial application. Compos B Eng 59:181–190
Anitha T, Senthil Kumar P, Sathish Kumar K (2015) Binding of Zn(II) ions to chitosan-PVA blend in aqueous environment: adsorption kinetics and equilibrium studies. Environ Prog Sustainable Energy 34:15–22
Annadurai G, Juang R-S, Lee DJ (2003) Adsorption of heavy metals from water using banana and orange peels. Water Sci Technol 47:185–190
Arbabi M, Hemati S, Amiri M (2015) Removal of lead ions from industrial wastewater: a review of Removal methods. Int J Epidemiol Res 2:105–109
Borai EH, Breky MME, Sayed MS, Abo-Aly MM (2015) Synthesis, characterization and application of titanium oxide nanocomposites for removal of radioactive cesium, cobalt, and europium ions. J Colloid Interface Sci 450:17–25
Brockmeyer B, Kraus UR, Theobald N (2015) Accelerated solvent extraction (ASE) for purification and extraction of silicone passive samplers used for the monitoring of organic pollutants. Environ Sci Pollut Res 22:19887–19895
Carter BA, Williams DB, Carter CB, Williams DB (1996) Transmission electron microscopy: a textbook for materials science. Diffraction. II. Springer Science & Business Media
Dakroury GA, El-Shazly EAA, Hassan HS (2021) Preparation and characterization of ZnO/Chitosan nanocomposite for Cs(I) and Sr(II) sorption from aqueous solutions. J Radioanal Nucl Chem 330:159–174
Dawood AS, Li Y (2014) Wastewater flocculation using a new hybrid copolymer: modeling and optimization by response surface methodology. Pol J Environ Stud 23:43–50
Dubey A, Mishra A, Singhal S (2014) Application of dried plant biomass as novel low-cost adsorbent for removal of cadmium from aqueous solution. Int J Environ Sci Technol 11:1043–1050
El-Aryan YF, Abdel-Galil EA, El-deen GES (2015) Synthesis, characterization and adsorption behavior of cesium, cobalt, and europium on organic-inorganic hybrid exchanger. Russ J Appl Chem 88:516–523
El-Dessouky MI, Ibrahiem HH, El-Masry EH et al (2018) Removal of Cs+ and Co2+ ions from aqueous solutions using poly (acrylamide-acrylic acid)/kaolin composite prepared by gamma radiation. Appl Clay Sci 151:73–80
El-Naggar IM, Mowafy EA, Abdel-Galil EA, El-Shahat MF (2010) Synthesis, characterization and ion-exchange properties of a novel ‘organic–inorganic’ hybrid cation-exchanger: polyacrylamide Sn(IV) molybdophosphate. Glob J Phys Chem 1:91–106
Ghasemi N, Ghasemi M, Moazeni S et al (2018) Zn(II) removal by amino-functionalized magnetic nanoparticles: Kinetics, isotherm, and thermodynamic aspects of adsorption. J Ind Eng Chem 62:302–310
Gupta AP, Agarwal H, Ikram S (2003) Studies on a new composites material polyaniline zirconium(IV) tungstophosphate-a thorium(IV) selective cation exchanger. J Indian Chem Soc 80:57–59
Gupta VK, Agarwal S, Tyagi I et al (2015) Synthesis, characterization and analytical application of cellulose acetate tin(IV) molybdate nanocomposite ion exchanger: binary separation of heavy metal ions and antimicrobial activity. Ionics 21:2069–2078
Gupta NK, Gupta A, Ramteke P et al (2019) Biosorption-a green method for the preconcentration of rare earth elements (REEs) from waste solutions: a review. J Mol Liq 274:148–164
Gürses A, Hassani A, Kıranşan M et al (2014) Removal of methylene blue from aqueous solution using by untreated lignite as potential low-cost adsorbent: kinetic, thermodynamic and equilibrium approach. J Water Process Eng 2:10–21
Hamed MM (2014) Sorbent extraction behavior of a nonionic surfactant, Triton X-100, onto commercial charcoal from low level-radioactive waste. J Radioanal Nucl Chem 302:303–313
Hamed MM, Holiel M, Ismail ZH (2016a) Removal of 134Cs and 152+154Eu from liquid radioactive waste using Dowex HCR-S/S. Radiochim Acta 104:399–413
Hamed MM, Rizk SE, Nayl AA (2016b) Adsorption kinetics and modeling of gadolinium and cobalt ions sorption by an ion-exchange resin. Part Sci Technol 34:716–724
Hamed MM, Shahr El-Din AM, Abdel-Galil EA (2019) Nanocomposite of polyaniline functionalized Tafla: synthesis, characterization, and application as a novel sorbent for selective removal of Fe(III). J Radioanal Nucl Chem 322:663–676. https://doi.org/10.1007/s10967-019-06733-0
Hamoud MA, Allan KF, Sanad WA et al (2014) Gamma irradiation induced preparation of poly(acrylamide–itaconic acid)/zirconium hydrous oxide for removal of Cs-134 radionuclide and methylene blue. J Radioanal Nucl Chem 302:169–178. https://doi.org/10.1007/s10967-014-3206-y
Hassan SSM, Kamel AH, Youssef MA et al (2020) Removal of barium and strontium from wastewater and radioactive wastes using a green bioadsorbent, Salvadora persica (Miswak). Desalin Water Treat 192:306–314
Hassan RS, Abass MR, Eid MA, Abdel-Galil EA (2021) Sorption of some radionuclides from liquid waste solutions using anionic clay hydrotalcite sorbent. Appl Radiat Isot 178:109985
Hassani A, Alidokht L, Khataee AR, Karaca S (2014) Optimization of comparative removal of two structurally different basic dyes using coal as a low-cost and available adsorbent. J Taiwan Inst Chem Eng 45:1597–1607
Herrera A, Tejada-Tovar C, González-Delgado ÁD (2020) Enhancement of cadmium adsorption capacities of agricultural residues and industrial fruit byproducts by the incorporation of Al2O3 nanoparticles. ACS Omega 5:23645–23653
Hurtado-Bermúdez S, Villa-Alfageme M, Mas JL, Alba MD (2018) Comparison of solvent extraction and extraction chromatography resin techniques for uranium isotopic characterization in high-level radioactive waste and barrier materials. Appl Radiat Isot 137:177–183
Ibrahim AB, Abass MR, EL-Masry EH, Abou-Mesalam MM (2021) Gamma radiation-induced polymerization of polyacrylic acid-dolomite composite and applications for removal of cesium, cobalt, and zirconium from aqueous solutions. Appl Radiat Isot 178:109956
Kalantari K, Ahmad MB, Masoumi HRF et al (2014) Rapid adsorption of heavy metals by Fe3O4/talc nanocomposite and optimization study using response surface methodology. Int J Mol Sci 15:12913–12927
Kang R, Qiu L, Fang L et al (2016) A novel magnetic and hydrophilic ion-imprinted polymer as a selective sorbent for the removal of cobalt ions from industrial wastewater. J Environ Chem Eng 4:2268–2277
Karaca S, Gürses A, Açışlı Ö et al (2013) Modeling of adsorption isotherms and kinetics of Remazol Red RB adsorption from aqueous solution by modified clay. Desalin Water Treat 51:2726–2739
Kayranli B (2021) Mechanism of interaction and removal of zinc with lignocellulosic adsorbents, closing the cycle with a soil conditioner. J King Saud Univ Sci 33:101607
Khataee A, Alidokht L, Hassani A, Karaca S (2013) Response surface analysis of removal of a textile dye by a Turkish coal powder. Adv Environ Res 2:291–308
Kulkarni SJ (2016) Research and studies on cobalt removal from wastewater. Int J Res Rev 2237:41–44
Lee S, Kim J, Ku B-C et al (2012a) Structural evolution of polyacrylonitrile fibers in stabilization and carbonization. Adv Chem Eng Sci 02:275–282. https://doi.org/10.4236/aces.2012.22032
Lee YK, Jeong JM, Hoigebazar L et al (2012b) Nanoparticles modified by encapsulation of ligands with a long alkyl chain to affect multispecific and multimodal imaging. J Nucl Med 53:1462–1470
Mehta P, Vedachalam S, Sathyaraj G et al (2020) Fast sensing ammonia at room temperature with proline ionic liquid incorporated cellulose acetate membranes. J Mol Liq 305:112820
Metwally SS, Hassan HS, Samy NM (2019) Impact of environmental conditions on the sorption behavior of 60Co and 152+154Eu radionuclides onto polyaniline/zirconium aluminate composite. J Mol Liq 287:110941
Mirzayev MN, Abdurakhimov BA, Demir E et al (2021) Investigation of the formation of defects under fast neutrons and gamma irradiation in 3C–SiC nano powder. Physica B 611:412842. https://doi.org/10.1016/j.physb.2021.412842
Nabi SA, Shahadat M, Bushra R et al (2011) Synthesis and characterization of nano-composite ion-exchanger; its adsorption behavior. Colloids Surf, B 87:122–128
Nilchi A, Atashi H, Javid AH, Saberi R (2007) Preparations of PAN-based adsorbers for separation of cesium and cobalt from radioactive wastes. Appl Radiat Isot 65:482–487. https://doi.org/10.1016/j.apradiso.2006.12.003
Park JH, Choppala G, Lee SJ et al (2013) Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water Air Soil Pollut 224:1–12
Pyrzynska K (2019) Removal of cadmium from wastewaters with low-cost adsorbents. J Environ Chem Eng 7:102795
Rahman N, Haseen U, Rashid M (2017) Synthesis and characterization of polyacrylamide zirconium(IV) iodate ion-exchanger: Its application for selective removal of lead(II) from wastewater. Arab J Chem 10:S1765–S1773. https://doi.org/10.1016/j.arabjc.2013.06.029
Sen TK, Gomez D (2011) Adsorption of zinc (Zn2+) from aqueous solution on natural bentonite. Desalination 267:286–294
Sheha RR, El-Zahhar AA (2008) Synthesis of some ferromagnetic composite resins and their metal removal characteristics in aqueous solutions. J Hazard Mater 150:795–803
Tan Y, Feng J, Qiu L et al (2017) The adsorption of Sr(II) and Cs(I) ions by irradiated Saccharomyces cerevisiae. J Radioanal Nucl Chem 314:2271–2280
Thi Huong N, Son NN, Phuong VH et al (2020) Synthesis Fe3O4/Talc nanocomposite by coprecipitation-ultrasonication method and advances in hexavalent chromium removal from aqueous solution. Adsorpt Sci Technol 38:483–501
Wang FY, Wang H, Ma JW (2010) Adsorption of cadmium(II) ions from aqueous solution by a new low-cost adsorbent Bamboo charcoal. J Hazard Mater 177:300–306
Wenlei L, Shanlin Z, Shuang C et al (2014) Adsorptive characteristics of modified talcum powder in removing methylene blue from wastewater. Chem Speciat Bioavailab 26:167–175
Zanazzi PF, Montagnoli M, Nazzareni S, Comodi P (2007) Structural effects of pressure on monoclinic chlorite: A single-crystal study. Am Miner 92:655–661
Zhang X, Hao Y, Wang X, Chen Z (2017) Rapid removal of zinc(II) from aqueous solutions using a mesoporous activated carbon prepared from agricultural waste. Materials 10:1002
Acknowledgements
This work has been supported by the Egyptian Atomic Energy Authority. Great thanks to all members of the Cyclotron project for helping us with the preparation of the material used.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.R. Abass: data curation, writing-original draft review, and editing. W.M. El-Kenany: experimental work and reviewing. E.H. EL-Masry: experimental work and editing.
Corresponding author
Ethics declarations
Ethics approval
Yes.
Consent to participate
Yes.
Consent to publish
Yes.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Gamma radiation at 50 kGy was used for the preparation of P(AA-AN)-talc.
• P(AA-AN)-talc was used for the removal of Cd(II), Co(II), Pb(II), Zn(II), and Sr(II).
• The saturation capacity was reduced by increasing heating temperatures.
• The thermodynamics showed an endothermic and spontaneous.
• P(AA-AN)-talc is promised for the column system to the recovery of investigated cations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abass, M.R., El-Kenany, W.M. & EL-Masry, E.H. High efficient removal of lead(II) and cadmium(II) ions from multi-component aqueous solutions using polyacrylic acid acrylonitrile talc nanocomposite. Environ Sci Pollut Res 29, 72929–72945 (2022). https://doi.org/10.1007/s11356-022-21023-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21023-1