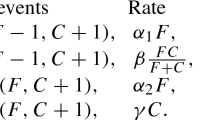Abstract
The reproductive cycle of mono-ovulatory species such as cows or humans is known to show two or more waves of follicular growth and decline between two successive ovulations. Within each wave, there is one dominant follicle escorted by subordinate follicles of varying number. Under the surge of the luteinizing hormone a growing dominant follicle ovulates. Rarely the number of ovulating follicles exceeds one. In the biological literature, the change of hormonal concentrations and individually varying numbers of follicular receptors are made responsible for the selection of exactly one dominant follicle, yet a clear cause has not been identified. In this paper, we suggest a synergistic explanation based on competition, formulated by a parsimoniously defined system of ordinary differential equations (ODEs) that quantifies the time evolution of multiple follicles and their competitive interaction during one wave. Not discriminating between follicles, growth and decline are given by fixed rates. Competition is introduced via a growth-suppressing term, equally supported by all follicles. We prove that the number of dominant follicles is determined exclusively by the ratio of follicular growth and competition. This number turns out to be independent of the number of subordinate follicles. The asymptotic behavior of the corresponding dynamical system is investigated rigorously, where we demonstrate that the \(\omega \)-limit set only contains fixed points. When also including follicular decline, our ODEs perfectly resemble ultrasound data of bovine follicles. Implications for the involved but not explicitly modeled hormones are discussed.





Similar content being viewed by others
Notes
The rare exception is known as superfecundation (Panza et al. 2016).
This also applies to ovulating follicles, which decline in size when releasing the oocyte, before transforming into a corpus luteum.
i.e., there are no limit cycles, etc.
References
Adams GP, Pierson RA (1995) Bovine model for study of ovarian follicular dynamics in humans. Theriogenology 43:113–120
Adams GP, Jaiswal R, Singh J, Malhi P (2008) Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 69:72–80
Baerwald AR, Adams GP, Pierson RA (2003) Characterization of ovarian follicular wave dynamics in women. Biol Reprod 69:1023–1031
Beam SW, Butler WR (1999) Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J Reprod Fertil Suppl 54:411–424
Bleach ECL, Glencross RG, Knight PG (2004) Association between ovarian follicle development and pregnancy rates in dairy cows undergoing spontaneous oestrous cycles. Reproduction 127:621–629
Boer HMT, Stötzel C, Röblitz S, Deuflhard P, Veerkamp RF, Woelders H (2011) A simple mathematical model of the bovine estrous cycle: follicle development and endocrine interactions. J Theor Biol 278:20–31
Burns DS, Jimenez-Krassel F, Ireland JLH, Knight PG, Ireland JJ (2005) Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod 73:54–62
Chavez-Ross A, Franks S, Mason HD, Hardy K, Stark J (1997) Modelling the control of ovulation and polycystic ovary syndrome. J Math Biol 36:95–118
Clement F, Monniaux D (2013) Multiscale modelling of ovarian follicular selection. Prog Biophys Mol Biol 113(3):398–408
Cummins SB, Lonergan P, Evans ACO, Butler ST (2012) Genetic merit for fertility traits in Holstein cows: II. Ovarian follicular and corpus luteum dynamics, reproductive hormones, and estrus behavior. J Dairy Sci 95:3698–3710
Drummond AE (2006) The role of steroids in follicular growth. Reprod Biol Endocrinol 4:16
Erickson GF, Wang C, Hsueh AJ (1979) FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature 279:336–338
Fortune JE (1994) Ovarian follicular growth and development in mammals. Biol Reprod 50:225–232
Fortune JE, Rivera GM, Evans ACO, Turzillo AM (2001) Differentiation of dominant versus subordinate follicles in cattle. Biol Reprod 65:648–654
Ginther OJ, Wiltbank MC, Fricke PM, Gibbons JR, Kot K (1996) Selection of the dominant follicle in cattle. Biol Reprod 55:1187–1194
Ginther OJ, Beg MA, Bergfelt DR, Donadeu FX, Kot K (2001) Follicle selection in monovular species. Biol Reprod 65:639–647
Gore MA, Nayudu PL, Vlaisavljevic V (1997) Attaining dominance in vivo: distinguishing dominant from challenger follicles in humans. Hum Reprod 12:2741–2747
Greenwald GS (1961) Quantitative study of follicular development in the ovary of the intact or unilaterally ovariectomized hamster. J Reprod Fertil 2:351–361
Guerra AG, Tribulo A, Yapura J, Adams GP, Singh J, Mapletoft RJ (2015) Lengthened superstimulatory treatment in cattle: evidence for rescue of follicles within a wave rather than continuous recruitment of new follicles. Theriogenology 84:467–476
Haughian JM, Ginther OJ, Diaz FJ, Wiltbank MC (2013) Gonadotropin-releasing hormone, estradiol, and inhibin regulation of follicle-stimulating hormone and luteinizing hormone surges: implications for follicle emergence and selection in heifers. Biol Reprod 88:1–10
Hendriksen PJM, Gadella BM, Vos PLAM, Mullaart E, Kruip TAM, Dieleman SJ (2003) Follicular dynamics around the recruitment of the first follicular wave in the cow. Biol Reprod 69:2036–2044
Iber D, De Geyter C (2013) Computational modelling of bovine ovarian follicle development. BMC Syst Biol 7:60
Kamel RM (2013) Assisted reproductive technology after the birth of Louise Brown. J Reprod Infertil 4:96–109
Kulick LJ, Bergfelt DR, Kot K, Ginther OJ (2001) Follicle selection in cattle: follicle deviation and codominance within sequential waves. Biol Reprod 65:839–846
Lacker HM (1981) Regulation of ovulation number in mammals. A follicle interaction law that controls maturation. Biophys J 35:433–454
Lacker HM, Percus A (1991) How do ovarian follicles interact? a many-body problem with unusual symmetry and symmetry-breaking properties. J Stat Phys 63:1133–1361
Lacker HM, Beers WH, Meuli LE, Akin E (1987) A theory of follicle selection: I. Hypotheses and examples; II. Computer simulation of estradiol administration in the primate. Biol Reprod 37:570–588
Lipschütz A (1928) New developments in ovarian dynamics and the law of follicular constancy. Br J Exp Biol 5:283–291
Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF (2004) Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab 89:5321–5327
Macklon NS, Fauser BCJM (2001) Follicle-stimulating hormone and advanced follicle development in the human. Arch Med Res 32:595–600
Mariana JC, Corpet F, Chevalet C (1994) Lacker’s model: control of follicular growth and ovulation in domestic species. Acta Biotheor 42:245–262
McGee EA, Hsueh AJW (2000) Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21:200–214
Michel P (2011) Multiscale modeling of follicular ovulation as a mass and maturity dynamical system. Multiscale Model Simul 9(1):282–313
Mihm M, Austin EJ (2002) The final stages of dominant follicle selection in cattle. Domest Anim Endocrinol 23:155–166
Mihm M, Evans ACO (2008) Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women. Reprod Dom Anim 43:48–56
Monniaux D, Michel P, Postel M, Clement F (2016) Multi-scale modelling of ovarian follicular development: from follicular morphogenesis to selection for ovulation. Biol Cell 108:149–160
Nagumo, M (1942) Über die Lage der Integralkurven gewöhnlicher Differentialgleichungen. In: Proceeding of the Physical–Mathematical Society Japan, vol 24, pp 551–559
Nishimoto H, Haman S, Hill GA, Miyamoto A, Tetsuka M (2009) Classification of bovine follicles based on the concentrations of steroids, glucose and lactate in follicular fluid and the status of accompanying follicles. J Reprod Dev 55:219–224
Panza NM, Wright AA, Selgrade JF (2016) A delay differential equation model of follicle waves in women. J Biol Dyn 10:1
Pring SR, Owen M, King JR, Sinclair KD, Webb R, Flint APF, Garnsworthy PC (2012) A mathematical model of the bovine oestrous cycle: simulating outcomes of dietary and pharmacological interventions. J Theor Biol 313:115–126
Sarty GE, Pierson RA (2005) An application of Lackers mathematical model for the prediction of ovarian response to superstimulation. Math Biosci 198(1):80–96
Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt M-A, Dupont J, Fortune JE, Gilchrist RB, Martin GB, McNatty KP, McNeilly AS, Monget P, Monniaux D, Violes C, Webb R (2011) Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev 23:444–467
Selgrade JF, Harris LA, Pasteur RD (2009) A model for hormonal control of the menstrual cycle: structural consistency but sensitivity with regard to data. J Theor Biol 260:572–580
Smith JF, Soboleva TK, Peterson AJ, Pleasants AB, Chagas LM, Burke CR (2005) Mathematical modelling of anoestrus in dairy cows and the linkage to nutrition. Proc N Z Soc Anim Prod 65:324–328
Soboleva TK, Peterson AJ, Pleasants AB, McNatty KP, Rhodes FM (2000) A model of follicular development and ovulation in sheep and cattle. Anim Reprod Sci 58:45–57
Soede NM, Langendijk P, Kemp B (2011) Reproductive cycles in pigs. Anim Reprod Sci 124:251–258
Stötzel C, Plöntzke J, Heuwieser W, Röblitz S (2012) Advances in modeling of the bovine estrous cycle: synchronization with PGF2\(\alpha \). Theriogenology 78:1415–1428
Webb R, Nicholas B, Gong JG, Campbell BK, Gutierrez CG, Garverick HA, Armstrong DG (2003) Mechanisms regulating follicular development and selection of the dominant follicle. Reprod Suppl 61:71–90
Wolfenson D, Inbar G, Roth Z, Kaim M, Bloch A, Braw-Tal R (2004) Follicular dynamics and concentrations of steroids and gonadotropins in lactating cows and nulliparous heifers. Theriogenology 62:1042–1055
Acknowledgements
We would like to thank Dr. S. Butler for sending us the data that have been used by Cummins et al. (2012). AL, JP, and SR gratefully acknowledge funding by Federal Ministry of Education and Research (BMBF) e:Bio Project BovSys (FKZ031A311). Furthermore, we thank the anonymous reviewers for their useful comments, which truly helped improving our manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Similar to follicle data and approximating curves of cow number 451 in Figs. 6 and 7 , follicle diameters of another cow (no. 3674) studied by Cummins et al. (2012) are shown in Fig. 8. Parameters and initial values of the ODEs, utilized to approximate follicle diameters over time (Figs. 6, 7, and 8), are provided in Tables 2 and 3 . The following constraints have been implemented: productive dimension \(\nu \le 3\), largest initial diameter \(x_1(t_0)\le 3\), and maximal diameter \(\xi \le 22\), if the corresponding wave is coupled to subsequent waves (where \(\lambda >0\)), or \(\xi \le 25\) otherwise (where \(\lambda =0\)).
Follicular kinetics with competition versus data. Follicle diameters of one cow (the same as on the l.h.s. panels in Fig. 3) during the first (l.h.s.) and second follicular wave (r.h.s.) are approximated by the two competitive growth models: logistic decline (14) in Panel (a) and simple decline (15) in Panel (b). The initial time for the second wave has been set to day 10. Six parameters (\(\eta ,\gamma ,\kappa ,\nu ,\rho ,\xi \); cf. Fig. 5) as well as three (l.h.s.) and four initial values (r.h.s.) have been determined to achieve the best possible least square fit, locally (cf. Tables 2 and 3 in the Appendix); RMSD indicates the root-mean-squared deviation. The good fitting result for the first wave with simple decline is due to the high \(\xi \) value, which (attained at the boundary of 25 mm) is higher than the possible follicle diameter. For the second wave, a better fit would be achievable as well if \(\xi \) was not restricted by 25 mm (cf. Appendix)
Follicular kinetics with competition and interaction between waves. Follicle diameters of one cow (the same as in Fig. 6) during the first (l.h.s.) and second follicular wave (r.h.s.) are approximated by the two competitive growth models including competitive coupling with subsequent waves (16): logistic decline (14) in Panel (a) and simple decline (15) in Panel (b). The initial time for the second wave has been set to day 10. Seven parameters (\(\eta ,\gamma ,\kappa ,\lambda ,\nu ,\rho ,\xi \)) as well as three (l.h.s.) and four initial values (r.h.s.) have been determined to achieve the best possible least square fit, locally (cf. Tables 2 and 3 in the Appendix); RMSD indicates the root-mean-squared deviation
Follicular kinetics. The eight panels show data and approximating curves of follicle diameters of cow no. 3674 studied by Cummins et al. (2012). Similar to Fig. 6, Panels (a) and (b) only include follicular interaction within one wave. Similar to Fig. 7, Panels (c) and (d) also incorporate interaction between waves. Panels (a) and (c) model logistic decline, Panels (b) and (d) simple decline. The l.h.s./r.h.s. panels illustrate follicles of the first/second wave, resp
Rights and permissions
About this article
Cite this article
Lange, A., Schwieger, R., Plöntzke, J. et al. Follicular competition in cows: the selection of dominant follicles as a synergistic effect. J. Math. Biol. 78, 579–606 (2019). https://doi.org/10.1007/s00285-018-1284-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-018-1284-0