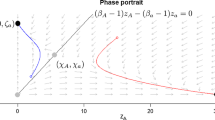
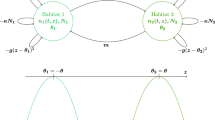
Abstract
Mechanisms leading to speciation are a major focus in evolutionary biology. In this paper, we present and study a stochastic model of population where individuals, with type a or A, are equivalent from ecological, demographical and spatial points of view, and differ only by their mating preference: two individuals with the same genotype have a higher probability to mate and produce a viable offspring. The population is subdivided in several patches and individuals may migrate between them. We show that mating preferences by themselves, even if they are very small, are enough to entail reproductive isolation between patches, and we provide the time needed for this isolation to occur as a function of the carrying capacity. Our results rely on a fine study of the stochastic process and of its deterministic limit in large population, which is given by a system of coupled nonlinear differential equations. Besides, we propose several generalisations of our model, and prove that our findings are robust for those generalisations.



Similar content being viewed by others
References
Abu Awad D, Billiard S (2017) The double edged sword: the demographic consequences of the evolution of self-fertilization. Evolution 71(5):1178–1190
Akerman A, Bürger R (2014) The consequences of gene flow for local adaptation and differentiation: a two-locus two-deme model. J Math Biol 68(5):1135–1198
Athreya KB, Ney PE (1972) Branching processes. Springer, Berlin
Avise JC, Mank JE (2009) Evolutionary perspectives on hermaphroditism in fishes. Sex Dev 3(2–3):152–163
Bank C, Bürger R, Hermisson J (2012) The limits to parapatric speciation: Dobzhansky–Muller incompatibilities in a continent–island model. Genetics 191(3):845–863
Bolker B, Pacala SW (1997) Using moment equations to understand stochastically driven spatial pattern formation in ecological systems. Theor Popul Biol 52(3):179–197. John Wiley & Sons, New York
Boughman JW (2001) Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411(6840):944–948
Boul KE, Funk WC, Darst CR, Cannatella DC, Ryan MJ (2007) Sexual selection drives speciation in an amazonian frog. Proc R Soc Lond B Biol Sci 274(1608):399–406
Bürger R, Schneider KA (2006) Intraspecific competitive divergence and convergence under assortative mating. Am Nat 167(2):190–205
Champagnat N (2006) A microscopic interpretation for adaptive dynamics trait substitution sequence models. Stoch Process Appl 116(8):1127–1160
Champagnat N, Ferrière R, Méléard S (2006) Unifying evolutionary dynamics: from individual stochastic processes to macroscopic models. Theor Popul Biol 69(3):297–321
Chaput-Bardy A, Grégoire A, Baguette M, Pagano A, Secondi J (2010) Condition and phenotype-dependent dispersal in a damselfly, Calopteryx splendens. PLoS ONE 5(5):e10.694
Chicone C (2006) Ordinary differential equations with applications, 2nd edn. Springer, New York. doi:10.1007/0-387-35794-7
Clobert J, Danchin E, Dhondt AA, Nichols JD (2001) Dispersal. Oxford University Press, Oxford
Collet P, Méléard S, Metz JAJ (2013) A rigorous model study of the adaptive dynamics of mendelian diploids. J Math Biol 67(3):569–607
Coron C (2015) Slow-fast stochastic diffusion dynamics and quasi-stationarity for diploid populations with varying size. J Math Biol 72(1–2):1–32
Coron C, Méléard S, Porcher E, Robert A (2013) Quantifying the mutational meltdown in diploid populations. Am Nat 181(5):623–636
Costa M, Hauzy C, Loeuille N, Méléard S (2015) Stochastic eco-evolutionary model of a prey-predator community. J Math Biol 72(3):573–622
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
Dieckmann U, Law R (2000) Relaxation projections and the method of moments. In: Dieckmann U, Law R, Metz JAJ (eds) The geometry of ecological interactions: symplifying spatial complexity. Cambridge University Press, Cambridge, pp 412–455
Ethier SN, Kurtz TG (1986) Markov processes: characterization and convergence. Wiley, New York
Fournier N, Méléard S (2004) A microscopic probabilistic description of a locally regulated population and macroscopic approximations. Ann Appl Probab 14(4):1880–1919
Freidlin M, Wentzell AD (1984) Random perturbations of dynamical systems, vol 260. Springer, New York
Gavrilets S (2003) Perspective: models of speciation: what have we learned in 40 years? Evolution 57(10):2197–2215
Gavrilets S (2004) Fitness landscapes and the origin of species. Princeton University Press, Princeton
Gavrilets S (2014) Models of speciation: where are we now? J Hered 105(S1):743–755
Gavrilets S, Boake CRB (1998) On the evolution of premating isolation after a founder event. Am Nat 152(5):706–716
Griffiths AJF, Miller JH, Suzuki DT, Lewontin RC, Gelbart WM (2000) An introduction to genetic analysis, 7th edn. W.H. Freeman, New-York
Haesler MP, Seehausen O (2005) Inheritance of female mating preference in a sympatric sibling species pair of Lake Victoria cichlids: implications for speciation. Proc R Soc Lond B Biol Sci 272(1560):237–245
Herrero M (2003) Male and female synchrony and the regulation of mating in flowering plants. Philos Trans R Soc B Biol Sci 358:1019–1024
Higashi M, Takimoto G, Yamamura N (1999) Sympatric speciation by sexual selection. Nature 402(6761):523–526
Hollocher H, Ting CT, Pollack F, Wu CI (1997) Incipient speciation by sexual isolation in drosophila melanogaster: variation in mating preference and correlation between sexes. Evolution 51(4):1175–1181
Höner OP, Wachter B, East ML, Streich WJ, Wilhelm K, Burke T, Hofer H (2007) Female mate-choice drives the evolution of male-biased dispersal in a social mammal. Nature 448:797–802
Jiang Y, Bolnick DI, Kirkpatrick M (2013) Assortative mating in animals. Am Nat 181(6):E125–E138
Jones AG, Ratterman NL (2009) Mate choice and sexual selection: what have we learned since darwin? PNAS 106(1):10,001–10,008
Kirkpatrick M (1982) Sexual selection and the evolution of female choice. Evolution 41:1–12
Kondrashov AS, Shpak M (1998) On the origin of species by means of assortative mating. Proc R Soc Lond B Biol Sci 265(1412):2273–2278
Kopp M, Hermisson J (2008) Competitive speciation and costs of choosiness. J Evolut Biol 21:1005–1023
Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci 78(6):3721–3725
Lande R, Kirkpatrick M (1988) Ecological speciation by sexual selection. J Theor Biol 133(1):85–98
LaSalle JP (1960) Some extensions of Liapunov’s second method. IRE Trans Circuit Theory 7(4):520–527
Leman H (2016) Convergence of an infinite dimensional stochastic process to a spatially structured trait substitution sequence. Stoch Partial Differ Equ Anal Comput 4(4):791–826
Matessi C, Gimelfarb A, Gavrilets S (2002) Long-term buildup of reproductive isolation promoted by disruptive selection: how far does it go? Selection 2(1–2):41–64
McLain DK, Boromisa RD (1987) Male choice, fighting ability, assortative mating and the intensity of sexual selection in the milkweed longhorn beetle, Tetraopes tetraophthalmus (coleoptera, cerambycidae). Behav Ecol Sociobiol 20(4):239–246
Mendelson TC, Shaw KL (2005) Sexual behaviour: rapid speciation in an arthropod. Nature 433(7024):375–376
Merrill RM, Wallbank RWR, Bull V, Salazar PCA, Mallet J, Stevens M, Jiggins CD (2012) Disruptive ecological selection on a mating cue. Proc R Soc Lond B Biol Sci 279(1749):4907–4913
M’Gonigle LK, Mazzucco R, Otto SP, Dieckmann U (2012) Sexual selection enables long-term coexistence despite ecological equivalence. Nature 484(7395):506–509
Nei M (1975) Molecular population genetics and evolution. North-Holland Publishing Company, Amsterdam
Neukirch R, Bovier A (2016) Survival of a recessive allele in a mendelian diploid model. J Math Biol 75(1):1–54
Otte D (1989) Speciation in hawaiian crickets. In: Otte D, Endler JA (eds) Speciation and its consequences. Sinauer, Sunderland, pp 482–526
Payne RJH, Krakauer DC (1997) Sexual selection, space, and speciation. Evolution 51(1):1–9
Pennings PS, Kopp M, Meszéna G, Dieckmann U, Hermisson J (2008) An analytically tractable model for competitive speciation. Am Nat 171(1):E44–E71
Ravigné V, Barberousse A, Bierne N, Britton-Davidian J, Capy P, Desdevises Y, Giraud T, Jousselin E, Moulia C, Smadja C et al (2010) La speciation. In: Thomas F, Lefèvre T, Raymond M (eds) Biologie Evolutive. De Boeck, pp 165–210
Ritchie MG (2007) Sexual selection and speciation. Annu Rev Ecol Evol Syst 38(1):79–102
Rudnicki R, Zwoleński P (2015) Model of phenotypic evolution in hermaphroditic populations. J Math Biol 70(6):1295–1321
Savolainen V, Anstett MC, Lexer C, Hutton I, Clarkson JJ, Norup MV, Powell MP, Springate D, Salamin N, Baker WJ (2006) Sympatric speciation in palms on an oceanic island. Nature 441:210–213
Schwagmeyer PL (1988) Scramble-competition polygyny in an asocial mammal: male mobility and mating success. Am Nat 131:885–892
Seehausen O, Van Alphen JJM, Witte F (1997) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277(5333):1808–1811
Seehausen O, Terai Y, Magalhaes I, Carleton K, Mrosso H, Miyagi R, van der Sluijs I, Schneider M, Maan M, Tachida H, Imai H, Okada N (2008) Speciation through sensory drive in cichlid fish. Nature 455(7213):620–626
Servedio MR (2010) Limits to the evolution of assortative mating by female choice under restricted gene flow. Proc R Soc Lond B Biol Sci 278(1703):179–187
Servedio MR, Bürger R (2014) The counterintuitive role of sexual selection in species maintenance and speciation. Proc Natl Acad Sci 111(22):8113–8118
Servedio MR, Bürger R (2015) The effects of sexual selection on trait divergence in a peripheral population with gene flow. Evolution 69(10):2648–2661
Shaw KL, Parsons YM (2002) Divergence of mate recognition behavior and its consequences for genetic architectures of speciation. Am Nat 159(S3):S61–S75
Smadi C (2015) An eco-evolutionary approach of adaptation and recombination in a large population of varying size. Stoch Process Appl 125(5):2054–2095
Turner GF, Burrows MT (1995) A model of sympatric speciation by sexual selection. Proc R Soc Lond B Biol Sci 260(1359):287–292
Van Doorn GS, Noest AJ, Hogeweg P (1998) Sympatric speciation and extinction driven by environment dependent sexual selection. Proc R Soc Lond B Biol Sci 265(1408):1915–1919
Van Doorn GS, Dieckmann U, Weissing FJ (2004) Sympatric speciation by sexual selection: a critical reevaluation. Am Nat 163(5):709–725
Weissing FJ, Edelaar P, Van Doorn GS (2011) Adaptive speciation theory: a conceptual review. Behav Ecol Sociobiol 65(3):461–480
Wu CI (1985) A stochastic simulation study on speciation by sexual selection. Evolution 39(1):66–82
Acknowledgements
The authors would like to warmly thank Sylvie Méléard for her continual guidance during their respective thesis works. They would also like to thank Pierre Collet for his help on the theory of dynamical systems, Sylvain Billiard for many fruitful discussions on the biological relevance of their model, Violaine Llaurens for her help during the revision of the manuscript, and the anonymous reviewers for their constructive comments that greatly contributed to improve the final version of the paper. C. C. and C. S. are grateful to the organizers of “The Helsinki Summer School on Mathematical Ecology and Evolution 2012: theory of speciation” which motivated this work. This work was partially funded by the Chair “Modélisation Mathématique et Biodiversité” of VEOLIA-Ecole Polytechnique-MNHN-F.X, and was also supported by a public grant as part of the Investissement d’avenir project, reference ANR-11-LABX-0056-LMH, LabEx LMH.
Author information
Authors and Affiliations
Corresponding author
A Technical results and reduction of the system
A Technical results and reduction of the system
This section is dedicated to some technical results needed in the proofs, as well as the reduction of the system to the minimal number of effective parameters. We first prove the convergence when K goes to infinity of the sequence of rescaled processes \(\mathbf {Z}^K\) to the solution of the dynamical system (7) stated in Lemma 1.
Proof (Proof of Lemma 1) The proof relies on a classical result presented in Chapter 11 of the book by Ethier and Kurtz (1986). Let \(\mathbf {z}\) be in \(\mathbb {N}^{\mathcal {E}}/K\). According to (2)–(5), the rescaled birth, death and migration rates
and
are Lipschitz and bounded on every compact subset of \( \mathbb {N}^{\mathcal {E}}\), and do not depend on the carrying capacity K.
Let \((Y_{\alpha ,i}^{(\lambda )},Y_{\alpha ,i}^{(d)},Y_{\alpha ,i}^{(\rho )},(\alpha ,i)\in {\mathcal {E}})\) be twelve independent standard Poisson processes. From the representation of the stochastic process \((\mathbf {N}^{K}(t),t\ge 0)\) in (6) we see that the stochastic process \((\bar{\mathbf {Z}}^{K}(t), t \ge 0)\) defined by
has the same law as \((\mathbf {Z}^{K}(t), t \ge 0)\). Moreover, a direct application of Theorem 2.1 p 456 in the book by Ethier and Kurtz (1986) gives that \((\bar{\mathbf {Z}}^{K}(t), t \le T)\) converges in probability to \((\mathbf {z}^{(\mathbf {z}^0)}(t), t \le T)\) for the uniform norm. As a consequence, \((\mathbf {Z}^K(t), t \le T)\) converges in law to \((\mathbf {z}^{(\mathbf {z}^0)}(t), t \le T)\) for the same norm. But the convergence in law to a constant is equivalent to the convergence in probability to the same constant. The result follows.
We now recall a well known fact on branching processes which can be found in the book by Athreya and Ney (1972), p. 109.
Lemma 4
-
Let \(Z=(Z_t)_{t \ge 0}\) be a birth and death process with individual birth and death rates b and d. For \(i \in \mathbb {Z}^+\), \(T_i=\inf \{ t\ge 0, Z_t=i \}\) and \(\mathbb {P}_i\) is the law of Z when \(Z_0=i\). If \(d\ne b \in \mathbb {R}_+^*\), for every \(i\in \mathbb {Z}_+\) and \(t \ge 0\),
$$\begin{aligned} \mathbb {P}_{i}(T_0\le t )= \Big ( \frac{d(1-e^{(d-b)t})}{b-de^{(d-b)t}} \Big )^{i}. \end{aligned}$$(68)
As we mentioned in Sect. 2, it is possible to reduce the number of parameters b, c, d, p, \(\beta \) by using a change of time and a scaling. Let us introduce the new variables
for all \(\alpha \in \{A,a\}\), \(i \in \{1,2\}\) and \(t \ge 0\), and the parameters
Then the new variables satisfy the following dynamical system
for \(\alpha \in \{A,a\}\), \(\bar{\alpha }=\{A,a\}\setminus \alpha \), \(i \in \{1,2\}\) and \(\bar{i}=\{1,2\} \setminus i\).
Rights and permissions
About this article
Cite this article
Coron, C., Costa, M., Leman, H. et al. A stochastic model for speciation by mating preferences. J. Math. Biol. 76, 1421–1463 (2018). https://doi.org/10.1007/s00285-017-1175-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-017-1175-9