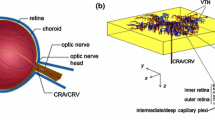
Abstract
The retina is the tissue layer at the back of the eye that is responsible for light detection. Whilst equipped with a rich supply of oxygen, it has one of the highest oxygen demands of any tissue in the body and, as such, supply and demand are finely balanced. It has been suggested that the protein neuroglobin (Ngb), which is found in high concentrations within the retina, may help to maintain an adequate supply of oxygen via the processes of transport and storage. We construct mathematical models, formulated as systems of reaction–diffusion equations in one-dimension, to test this hypothesis. Numerical simulations show that Ngb may play an important role in oxygen transport, but not in storage. Our models predict that the retina is most susceptible to hypoxia in the regions of the photoreceptor inner segment and inner plexiform layers, where Ngb has the potential to prevent hypoxia and increase oxygen uptake by 30–40 %. Analysis of a simplified model confirms the utility of Ngb in transport and shows that its oxygen affinity (\(P_{50}\) value) is near optimal for this process. Lastly, asymptotic analysis enables us to identify conditions under which the piecewise linear and quadratic approximations to the retinal oxygen profile, used in the literature, are valid.












Similar content being viewed by others
References
Alder VA, Cringle SJ, Constable IJ (1983) The retinal oxygen profile in cats. Invest Ophthalmol Vis Sci 24(1):30-36
Anderson B (1968) Ocular effects of changes in oxygen and carbon dioxide tension. Trans Am Ophthalmol Soc 66:423-474
Anderson B, Saltzman HA (1964) Retinal oxygen utilization measured by hyperbaric blackout. Arch Ophthalmol 72(6):792-795
Bender CM, Orszag SA (1999) Advanced mathematical methods for scientists and engineers I: asymptotic methods and perturbation theory. Springer, Berlin
Bentmann A, Schmidt M, Reuss S, Wolfrum U, Hankeln T, Burmester T (2005) Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J Biol Chem 280(21):20660-20665
Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA (2007) Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol 293(3):H1696-H1704
Braun RD, Linsenmeier RA, Goldstick TK (1995) Oxygen consumption in the inner and outer retina of the cat. Invest Ophthalmol Vis Sci 36(3):542-554
Burmester T, Hankeln T (2004) Neuroglobin: a respiratory protein of the nervous system. News Physiol Sci 19(3):110-113
Burmester T, Hankeln T (2009) What is the function of neuroglobin? J Exp Biol 212(10):1423-1428
Burmester T, Weich B, Reinhardt S, Hankeln T (2000) A vertebrate globin expressed in the brain. Nature 407(6803):520-523
Burmester T, Ebner B, Weich B, Hankeln T (2002) Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol 19(4):416-421
Chan G, Balaratnasingam C, Yu PK, Morgan WH, McAllister IL, Cringle SJ, Yu DY (2012) Quantitative morphometry of perifoveal capillary networks in the human retina. Invest Ophthalmol Vis Sci 53(9):5502-5514
Costa LE, Mendez G, Boveris A (1997) Oxygen dependence of mitochondrial function measured by high-resolution respirometry in long-term hypoxic rats. Am J Physiol 273(3):C852-C858
Cringle SJ, Yu DY (2002) A multi-layer model of retinal oxygen supply and consumption helps explain the muted rise in inner retinal PO(2) during systemic hyperoxia. Comp Biochem Physiol 132(1):61-66
Dollery CT, Bulpitt CJ, Kohner EM (1969) Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions. Invest Ophthalmol Vis Sci 8(6):588-594
Fago A, Hundahl C, Malte H, Weber RE (2004b) Functional properties of neuroglobin and cytoglobin. insights into the ancestral physiological roles of globins. IUBMB Life 56(11-12):689-696
Gillies MC, Su T, Naidoo D (1995) Electrical resistance and macromolecular permeability of retinal capillary endothelial cells in vitro. Curr Eye Res 14(6):435-442
Goldman D (2008) Theoretical models of microvascular oxygen transport to tissue. Microcirculation 15(8):795-811
Hamdane D, Kiger L, Dewilde S, Green BN, Pesce A, Uzan J, Burmester T, Hankeln T, Bolognesi M, Moens L, Marden MC (2003) The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J Biol Chem 278(51):51713-51721
Hardarson SH, Stefánsson E (2010) Oxygen saturation in central retinal vein occlusion. Am J Ophthalmol 150(6):871-875
Hardarson SH, Basit S, Jonsdottir TE, Eysteinsson T, Halldorsson GH, Karlsson RA, Beach JM, Benediktsson JA, Stefansson E (2009) Oxygen saturation in human retinal vessels is higher in dark than in light. Invest Ophthalmol Vis Sci 50(5):2308-2311
Haugh L, Linsenmeier R, Goldstick T (1990) Mathematical models of the spatial distribution of retinal oxygen tension and consumption, including changes upon illumination. Ann Biomed Eng 18:19-36
Jürgens KD, Peters T, Gros G (1994) Diffusivity of myoglobin in intact skeletal muscle cells. Proc Natl Acad Sci 91(9):3829-3833
Keener J, Sneyd J (1998) Mathematical physiology. Springer, Berlin
Kiger L, Uzan J, Dewilde S, Burmester T, Hankeln T, Moens L, Hamdane D, Baudin-Creuza V, Marden MC (2004) Neuroglobin ligand binding kinetics. IUBMB Life 56(11-12):709-719
Kohen R, Nyska A (2002) Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30(6):620-650
Kur J, Newman EA, Chan-Ling T (2012) Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res 31(5):377-406
Linsenmeier RA (1986) Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol 88(4):521-542
Linsenmeier RA, Braun RD (1992) Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J Gen Physiol 99(2):177-197
Linsenmeier RA, Padnick-Silver L (2000) Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci 41(10):3117-3123
Linsenmeier RA, Yancey CM (1989) Effects of hyperoxia on the oxygen distribution in the intact cat retina. Invest Ophthalmol Vis Sci 30(4):612-618
McGuire BJ, Secomb TW (2001) A theoretical model for oxygen transport in skeletal muscle under conditions of high oxygen demand. J Appl Physiol 91(5):2255-2265
Ockendon J, Howison S, Lacey A, Movchan A (2003) Applied partial differential equations, revised edn. Oxford University Press, Oxford
Ostojić J, Sakaguchi DS, de Lathouder Y, Hargrove MS, Trent JT, Kwon YH, Kardon RH, Kuehn MH, Betts DM, Grozdanić S (2006) Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest Ophthalmol Vis Sci 47(3):1016-1023
Ostojić J, Grozdanić SD, Syed NA, Hargrove MS, Trent JT, Kuehn MH, Kwon YH, Kardon RH, Sakaguchi DS (2008) Patterns of distribution of oxygen-binding globins, neuroglobin and cytoglobin in human retina. Arch Ophthalmol 126(11):1530-1536
Oyster CW (1999) The human eye: structure and function. Sinauer Associates Inc, Sunderland
Padnick-Silver L, Linsenmeier RA (2003) Effect of acute hyperglycemia on oxygen and oxidative metabolism in the intact cat retina. Invest Ophthalmol Vis Sci 44(2):745-750
Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E (2008) Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 27(3):284-330
Rajendram R, Rao NA (2007) Neuroglobin in normal retina and retina from eyes with advanced glaucoma. Br J Ophthalmol 91(5):663-666
Richmond KN, Shonat RD, Lynch RM, Johnson PC (1999) Critical PO(2) of skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 277(5):H1831-H1840
Roh HD, Goldstick TK, Linsenmeier RA (1990) Spatial variation of the local tissue oxygen diffusion coefficient measured in situ in the cat retina and cornea. Adv Exp Med Biol 277:127-136
Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T (2003) How does the eye breathe? J Biol Chem 278(3):1932-1935
Schmidt M, Laufs T, Reuss S, Hankeln T, Burmester T (2005) Divergent distribution of cytoglobin and neuroglobin in the murine eye. Neurosci Lett 374(3):207-211
Stefánsson E (1988) Retinal oxygen tension is higher in light than dark. Pediatr Res 23:5-8
Swaroop A, Kim D, Forrest D (2010) Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci 11:563-576
Tan PEZ, Yu PK, Balaratnasingam C, Cringle SJ, Morgan WH, McAllister IL, Yu DY (2012) Quantitative confocal imaging of the retinal microvasculature in the human retina. Invest Ophthalmol Vis Sci 53(9):5728-5736
Törnquist P, Alm A, Bill A (1990) Permeability of ocular vessels and transport across the blood-retinal-barrier. Eye 4(2):303-309
Trent JT, Hargrove MS (2002) A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem 277(22):19538-19545
Wangsa-Wirawan ND, Linsenmeier RA (2003) Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 121(4):547-557
Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM (1988) The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem 263(6):2712-2718
Yu DY, Cringle SJ (2001) Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res 20(2):175-208
Yu DY, Cringle SJ (2002) Outer retinal anoxia during dark adaptation is not a general property of mammalian retinas. Comp Biochem Physiol 132(1):47-52
Yu DY, Cringle SJ (2005) Retinal degeneration and local oxygen metabolism. Exp Eye Res 80(6):745-751
Yu DY, Cringle SJ, Alder VA, Su EN (1994) Intraretinal oxygen distribution in rats as a function of systemic blood pressure. Am J Physiol Heart Circ Physiol 267(6):H2498-H2507
Yu DY, Cringle SJ, Alder V, Su EN (1999) Intraretinal oxygen distribution in the rat with graded systemic hyperoxia and hypercapnia. Invest Ophthalmol Vis Sci 40(9):2082-2087
Yu DY, Cringle SJ, Su EN, Yu PK (2000) Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci 41(12):3999-4006
Yu DY, Cringle S, Valter K, Walsh N, Lee D, Stone J (2004) Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest Ophthalmol Vis Sci 45(6):2013-2019
Yu DY, Cringle SJ, Su EN (2005) Intraretinal oxygen distribution in the monkey retina and the response to systemic hyperoxia. Invest Ophthalmol Vis Sci 46(12):4728-4733
Acknowledgments
We gratefully acknowledge the Engineering and Physical Sciences Research Council (EPSRC) in the UK for funding through a studentship at the Systems Biology programme of the University of Oxford’s Doctoral Training Centre P.A.R.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Placing a bound on the concentration of pentacoordinate neuroglobin
It was found in all single and eight layer model simulations that the concentration of pentacoordinate Ngb, n, at any given point in space is significantly lower than that of hexacoordinate Ngb, \(n_h\), or oxygen bound Ngb, \(n_o\), at that point. In this appendix we derive a bound on n to explain why this is the case.
Beginning with the time-dependent equation for n, Eq. (10), we can re-write it in the form
where \(1/\kappa = k_3\), \(f(x,t) = k_4n_h + \alpha k_2n_o \ge 0\) and \(g(x,t) = \alpha k_1 c \ge 0\). Whether we are using the single or the eight layer model, this equation will be defined on a finite domain, which we can denote as \(x \in [0,L]\) without loss of generality. As usual, we impose zero-flux boundary conditions at either end of the domain.
Consider the function \(M = M(t)\) which satisfies
where the constant \(\beta > 0\) is arbitrarily small. This can be written as
where \(F = (\max _{x,t}(f(x,t)) + \beta ) > f(x,t) \ge 0\) is a constant. We close the system by imposing the initial condition
Solving this initial-value problem using the integrating factor method we obtain
Taking the limit as t tends to infinity we find that
Let \(u(x,t) := n(x,t) - M(t)\), then
from Eqs. (42) and (44). Since \((f - F) < 0\) and \(gn \ge 0\) we have that
and therefore
Defining \(v(x,t) := e^{\frac{t}{\kappa }}u(x,t)\), we have that
with initial condition
and zero-flux boundary conditions.
By the maximum principle for parabolic PDEs we have that v must achieve its maximum on one of the boundaries \(x = 0\), \(x = L\) or \(t = 0\) (Ockendon et al. 2003). Suppose that v takes its maximum value on \(x = 0\) for some \(t = t_1 > 0\). Since \(n_x(0,t_1) = 0\) by the zero-flux boundary condition and since \(M_x(t_1) = 0\), as M is independent of x, this implies that \(u_x(0,t_1) = 0\) and so \(v_x(0,t_1) = 0\). Therefore, in order for this point to be a maximum, we require both that \(v_t(0,t_1) = 0\) and \(v_{xx}(0,t_1) \le 0\). This implies that \(v_t(0,t_1) - Dv_{xx}(0,t_1) \ge 0\), which contradicts (51). Therefore, the maximum cannot lie on the boundary \(x = 0\). Using a similar argument we can show that the maximum cannot lie on the boundary \(x = L\). Therefore, the maximum must lie on the boundary \(t = 0\). As a check we can see that if the maximum lies on the boundary \(t = 0\) for some \(x = x_1\), where \(0 < x_1 < L\), then we must have that \(v_t(x_1,0) \le 0\), \(v_x(x_1,0) = 0\) and \(v_{xx}(x_1,0) \le 0\). Thus (51) may be satisfied, provided \(|v_t(x_1,0)| > D|v_{xx}(x_1,0)|\).
Since v(x, t) takes its maximum value on \(t = 0\) this means that \(v(x,t) \le \max _{x}(v(x,0)) = \max _{x}(n(x,0) - \max _{x}(n(x,0))) = \max _{x}(n(x,0)) - \max _{x}(n(x,0)) = 0\). Therefore \(u \le 0\), which implies that \(n \le M\). Therefore, by (47), we have that at steady-state \(n \le \kappa F\). Taking the limit as \(\beta \) tends to zero, we find \(n \le \kappa \max _{x,t}(f(x,t)) = \kappa \max _{x,t}(k_4n_h + \alpha k_2n_o) \le \kappa \alpha k_2 n_T = \frac{\alpha k_2 n_T}{k_3} \approx 4n_T\times 10^{-4}\). This bound was found to be satisfied in all simulations.
Appendix 2: Asymptotic analysis
In the absence of Ngb, Eqs. (21)–(23) reduce to a single equation for oxygen:
for \(Q_i\) \((1,\ldots ,7^*).\)
In layers 2 and 4, \(Q_2 = Q_4 = 0\), so that an exact analytical solution to Eq. (53) may be obtained. In all other layers, \(Q_i\) is strictly positive, so that an exact analytical solution cannot be derived. In these layers we look instead for a leading order solution. Since the value of \(Q_i\) is not, in general, continuous between layers, we cannot use standard matching techniques to construct a solution which is valid across all layers. Instead we use patching, ensuring that the solution in each layer satisfies the boundary conditions between it and the adjoining layers (see Bender and Orszag 1999, pgs. 335–336 for a discussion of patching).
We construct an asymptotic expansion for c(x):
choosing \(\varepsilon = 0.1\), so as to provide a clear separation between the various scales, and introduce \(\gamma ^* = \varepsilon ^{-2} \gamma \) so that \(\gamma ^* = O(1)\). We also note that \(Q_i = O(1)\) for \(i \in \{1,3,5,6^*,7^*\}\) and that \(Q_2 = Q_4 = 0\). Applying the scaling on \(\gamma \), Eq. (53) becomes
We now consider the leading order solution to Eq. (55) within each layer, grouping layers that have the same scaling.
1.1 Layer 1
In layer 1, \(c = O(1)\), as can be seen in Fig. 9, and Eq. (55) supplies, at leading order,
where \(A_1\) and \(B_1\) are constants.
1.2 Layers 2 and 4
In layers 2 and 4, \(Q_2 = Q_4 = 0\), so that Eq. (55) can be solved exactly to yield
for \(i = 2, 4,\) where \(A_i\) and \(B_i\) are constants.
1.3 Layers 3, 5 and 7\(^*\)
In layers 3, 5 and 7\(^*\), \(c = O(\varepsilon )\), as can be seen in Fig. 9. Rescaling oxygen as \(c = \varepsilon c^*\), we then rescale x as \(x = \varepsilon ^{1/2}x_i^* + L_{i-1}\) in order to achieve a dominant balance, so that, after returning to our original scaling on c and x we have, at leading order,
for \(i \in \{3,5,7^*\}\), where \(A_i\) and \(B_i\) are constants.
1.4 Layer 6\(^*\)
The situation in layer 6\(^*\) is less straightforward. On the left- and right-hand sides of layer 6\(^*\), \(c = O(\varepsilon )\), whereas, toward the centre of the layer, in a neighbourhood around the local minimum, \(c = O(\varepsilon ^2)\). Where \(c = O(\varepsilon )\), we could scale c and x as in layers 3, 5 and 7\(^*\); however, in the central region, where \(c = O(\varepsilon ^2)\), we would regain Eq. (53) in the dominant balance after dropping the stars. Since seeking leading order solutions does not allow us to avoid dealing with Eq. (53), we instead retain Eq. (53) across the whole of layer 6\(^*\) and use quadrature methods to derive approximate analytical solutions for the oxygen profile in this layer.
We will derive separate approximations to the oxygen profile for the left-hand side, centre and right-hand side of layer 6\(^*\), in order to account for the variation in c between \(O(\varepsilon )\) and \(O(\varepsilon ^2)\). We will also derive approximations to the minimum oxygen concentration in layer 6\(^*\), \(c_{min}\), and its position, \(x_{min}\).
Multiplying Eq. (53) by \({\mathrm {d}}c/{\mathrm {d}}x\) and integrating between \(x_{min}\) and x, we find that
where we take the positive (negative) root to the right (left) of \(x_{min}\), since the gradient of the oxygen profile is positive (negative) there. We note that the values of \(c_{min}\) and \(x_{min}\) are unknown at this stage.
We begin by seeking the left-hand and right-hand approximations near \(L_5\) and \(L_{6^*}\) respectively. For the left-hand approximation, we integrate Eq. (59) between x and \(L_5\) to obtain
where \(c_L = c(x=L_5)\) is unknown at this stage. Since \(c \approx c_L\) in the left-hand region, where \(c_L = O(\varepsilon )\), and since \(c_{min} = O(\varepsilon ^2)\) and \(\gamma = O(\varepsilon ^2)\) (see Fig. 9 for values of \(c_L\) and \(c_{min}\)), we may approximate the integrand by \(s^{-\frac{1}{2}}\) to obtain the left-hand approximation as
In a similar way, we obtain the right-hand approximation:
where \(c_R = c(x=L_{6^*})\) is unknown at this stage. Both \(c_L\) and \(c_R\) may be found by applying the boundary conditions as described at the end of this section.
To derive the central approximation, valid in the neighbourhood of \(x_{min}\), we integrate Eq. (59) between \(x_{min}\) and x. Writing \(\log ((\gamma + s)/(\gamma + c_{min})) = \log (1 + (s - c_{min})/(\gamma + c_{min}))\), we expand the integrand (which is the same as in Eq. (60)) about \(s = c_{min}\), in powers of \((s - c_{min})/(\gamma + c_{min})\), where s is the variable of integration and \(|(s - c_{min})/(\gamma + c_{min})| \ll 1\). Retaining only the first term and neglecting higher order terms, we obtain the central solution:
in which \(x_{min}\) and \(c_{min}\) are presently unknown.
To determine \(x_{min}\), we integrate Eq. (59) between the limits \(x_{min}\) and \(L_5\), and \(x_{min}\) and \(L_{6^*}\), to obtain the following pair of equations:
Subtracting Eq. (64) from Eq. (65), and noting that \(\min (c_L,c_R) < s < \max (c_L,c_R)\) in the integrand, where \(c_L = O(\varepsilon )\), \(c_R = O(\varepsilon )\), \(c_{min} = O(\varepsilon ^2)\) and \(\gamma = O(\varepsilon ^2)\) (see Fig. 9 for values of \(c_L\), \(c_R\) and \(c_{min}\)), we may approximate the integrand by \(s^{-\frac{1}{2}}\) to obtain
We obtain an implicit expression for \(c_{min}\), by substituting for \(x_{min}\) from Eq. (66) into Eq. (64):
where the integral must be calculated numerically. With \(x_{min}\) and \(c_{min}\) specified by (66) and (67) respectively, we can use Eq. (63) to calculate the central solution.
1.5 Using iteration to improve accuracy
We can iteratively improve the accuracy of our approximation using the exact derivative, given by Eq. (59), in the oxygen-flux boundary conditions at \(x=L_{6^*}\) and \(x=L_{7^*}\), given by Eq. (20), to yield:
The constant \(c_{min}\) takes the value calculated by solving (67), using the original values of \(c_L\) and \(c_R\), whilst the constant \(A_5\) is a function of \(c_L\), given by:
Equations (68)–(69) can be solved numerically, using the Matlab routine fsolve, to find updated values for \(c_L\) and \(c_R\). The updated \(c_L\) and \(c_R\) can then be used to calculate an updated value for \(c_{min}\), using Eq. (67). We may then repeat the iteration, using the updated value of \(c_{min}\) in Eqs. (68)–(70), to find new values for \(c_L\) and \(c_R\). Once the solution has converged, the final values of \(c_L\) and \(c_R\) can then be used to calculate improved values for \(A_1,\ldots ,A_5,B_{7^*},x_{min}\) and \(c_{min}\), by applying the boundary conditions to Eq. (41) as described in Sect. 4.3 and using Eqs. (66) and (67).
It was found that iteration results in a small improvement in the accuracy of the approximation and that the solution does not change significantly (by \(O(10^{-4})\) or greater) after the third iteration. Therefore, we use the parameters generated by the third iteration for the approximate solution in Sect. 4.3.
Rights and permissions
About this article
Cite this article
Roberts, P.A., Gaffney, E.A., Luthert, P.J. et al. Retinal oxygen distribution and the role of neuroglobin. J. Math. Biol. 73, 1–38 (2016). https://doi.org/10.1007/s00285-015-0931-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-015-0931-y