Abstract
Antimicrobial susceptibility testing (AST) by disk diffusion provides an accurate image of bacterial growth, enabling the detection of culture purity, heterogeneous growth, and antibiotic interactions. However, this manual method is time-consuming and visual interpretation is prone to errors. To overcome these disadvantages, the Radian® In-Line Carousel (Copan, Brescia, Italy) was launched, which is a WASPLab® module dedicated to full automation of (pre)-analytical steps as well as interpretation of disk diffusion AST. However, until now, no evaluation of Radian® against manual disk diffusion has been performed. We assessed the categorical agreement (CA) between standardized disk diffusion (reference method) and Radian® using EUCAST 2021 breakpoints. We tested 135 non-duplicate strains, selected from the National EUCAST challenge panel, clinical strains, and external quality controls. The strains included Enterobacterales (n = 63), Enterococcus faecalis (n = 3), Enterococcus faecium (n = 10), Pseudomonas aeruginosa (n = 16), Staphylococcus aureus (n = 19), coagulase-negative staphylococci (n = 4), and Streptococcus spp. (n = 20). Furthermore, we explored antibiotic disk thermolability in the WASP Radian® carousel by testing 10 ATCC® strains up to 7 days. The observed CA was 95.3%, 96.3%, 93.8%, 97.3% and 98.0% for Enterobacterales, Enterococcus spp., P. aeruginosa, Staphylococcus spp. and Streptococcus spp., respectively, resulting in an acceptable overall CA for all groups. (Very) major error rates were ≤ 5% for all antibiotics. Antibiotic disk thermostability was confirmed up to 4 days in the WASP Radian® In-Line Carousel. The Radian® In-Line Carousel provides a fully automated solution for accurate disk diffusion AST, reducing workload and improving standardization and traceability. In addition, our study demonstrated the thermostability of antibiotic disks up to 4 days in the WASP Radian® In-Line Carousel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identifying the causative bacteria of an infection and performing antimicrobial susceptibility testing (AST) to determine a susceptibility profile are crucial assignments in clinical microbiology laboratories. The AST profile enables good antibiotic prescribing, optimal patient care, and prevention of bacterial resistance by initiating appropriate escalation or de-escalation of antibiotics [1,2,3]. Different AST methods utilize the presence or absence of bacterial growth on a solid agar plate or liquid growth medium containing antibiotics to determine the sensitivity of the bacteria [4, 5]. Disk diffusion testing, a commonly used AST method in routine clinical microbiology laboratories, provides a true in vitro image of bacterial growth, enabling the detection of culture purity, heterogeneous growth, and antibiotic interactions [5,6,7]. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) developed a standardized AST method based on disk diffusion with zone diameter breakpoints correlated with clinical minimal inhibitory concentration (MIC) breakpoints [8]. Disk diffusion is a time-consuming method that requires 16–20 h of incubation to measure inhibition zones. Semi-automated reading of zone diameters can be conducted using SIRScan® (i2a, Montpellier, France), but is still prone to interpretation errors [3, 9].
Designing and implementing laboratory automation systems plays a crucial role in improving productivity, traceability, and quality and is an ongoing process [2, 7, 10]. One such automation system is WASPLab® (Copan, Brescia, Italy), which enables automated inoculation of specimens, incubation of solid plates, and analysis of bacterial cultures through images [11, 12]. Additionally, Copan has developed the Radian® in-line Carousel and Radian® expert System to complement WASPLab® by automating antimicrobial disk diffusion testing and interpretation [2, 3]. It contains a carousel that is capable of holding up to 50 antimicrobial discs. Laboratories are able to customize the combination and location of antimicrobial discs that are placed on the prepared Mueller Hinton plates. In combination with WASPLab® the prepared agars are automatically transferred to the incubators. Following the predefined incubation periods, plate imaging occurs automatically. These images are then processed by the Radian® expert system which performs zone measurement and interpretation.
According to EUCAST and manufacturers’ guidelines, antibiotic disks should be stored in sealed containers, protected from humidity and light, at a temperature lower than 8 °C when not in use. Before use, the antibiotic disks should reach room temperature, and the sealed container should not be opened before this temperature is reached to avoid humidity [6]. Radian® is not equipped with a cooling device, so placing the antibiotic disks at room temperature for long periods when being stored inside. Due to the practical disadvantage of needing to retract up to 50 antibiotic disks from Radian® at the end of each working day and placing them in the fridge, a thermolability study was performed.
We evaluated the automation of disk diffusion AST using WASPLab® with Radian® In-Line Carousel (Copan) against manual disk diffusion with semi-automated reading by SIRScan® (i2a).
Materials and Methods
Strains
Antimicrobial Susceptibility Testing
135 non-duplicate strains were tested, selected from the National EUCAST challenge panel (n = 28) [13], clinical strains (n = 37) and external quality controls (n = 70) conserved at −80 °C: Enterobacterales n = 63, Enterococcus faecalis n = 3, Enterococcus faecium n = 10, Pseudomonas aeruginosa n = 16, Staphylococcus aureus n = 19, coagulase negative staphylococci n = 4 and Streptococcus spp. n = 20 (Streptococcus pneumoniae n = 10). Different resistance mechanisms were included in this study: 13 extended-spectrum β-lactamase (ESBL)—producing Enterobacterales (Escherichia coli n = 7, Klebsiella oxytoca n = 1, Klebsiella pneumoniae n = 5), 9 carbapenemase producing Enterobacterales (CPE) (VIM-1 producing Enterobacter cloacae complex n = 1 and K. pneumoniae n = 1, OXA-48 producing Escherichia coli n = 1 and K. pneumoniae n = 4, KPC producing K. pneumoniae n = 2), 3 metallo-beta-lactamase (MBL) producing P. aeruginosa (VIM-2 n = 2), 10 vancomycin-resistant enterococci (VRE) (VanB positive E. faecalis n = 1, VanA positive E. faecium n = 4, VanB positive E. faecium n = 4 and one E. faecium with unknown Van genes) and 15 methicillin-resistant S. aureus (MRSA).
Thermolability of Antibiotic Disks
10 reference strains, conserved at −80 °C, were tested: E. coli ATCC® 25922™ and ATCC® 35218™, P. aeruginosa ATCC® 27853™, K. pneumoniae ATCC® 700603™, S. aureus ATCC® 29213™ and NCTC® 12493™, E. faecalis ATCC® 29212™ and ATCC® 51299™, S. pneumoniae ATCC® 49619™ and Campylobacter jejuni ATCC® 33560™.
AST Methodology
All strains were processed in parallel in two ways: (i) manually according to EUCAST [8] and (ii) using WASPLab® and Radian® in-Line Carousel. Strains were cultured manually on a non-selective blood agar (Thermo Fisher Scientific, Waltham, USA). These agar plates were incubated for 16 h in a CO2 incubator (Thermo Fisher Scientific). Species were identified by MALDI Biotyper® Sirius system (Bruker Daltonics, Bremen, Germany).
EUCAST Standardized Disk Diffusion Testing
Standardized disk diffusion testing was performed according to EUCAST methodology [8]. A 0.5 McFardland (McF) solution was made starting from freshly grown colonies in 0.9% saline solution. Four drops of this inoculum were transferred to a 120 mm square Mueller–Hinton (MH) agar (Axonlab, Hengersberg, Germany) and streaking was done manually followed by the addition of 16 antibiotic disks (i2a) with a disk dispenser. For S. pneumoniae MH-F agars (Thermo Fisher Scientific) were used as recommended by EUCAST. The MH(-F) agars were incubated for 18 ± 2 h, aerobic for MH-agars and CO2-enriched for MH-F agars (Thermo Fisher Scientific). Inhibition zones were read using SIRScan 2000 (i2a) [14]. The inhibition zones were manually adjusted when necessary.
WASPLab®/Radian® In-Line Carousel
AST by WASPLab® and Radian® In-Line Carousel was performed according to EUCAST guidelines for standardized disk diffusion adapted for inoculation by WASP® corresponding with manufacturer’s instructions [15]. A 0.5 McF solution was made starting from freshly grown colonies in 0.9% saline solution. A 1/3 dilution of this suspension in phosphate-buffered saline was made, which was further used as inoculum. 60 µL of this inoculum was applied by WASP® to three, 90 mm circular MH agars (Thermo Fisher Scientific). Using the Radian® In-Line Carousel, six antibiotic disks (Thermo Fisher Scientific) were dispensed to each MH-agar for the Gram-negative strains. For the Gram-positive strains, six antibiotic disks were dispensed on two MH-agars and four on a third MH-agar. For S. pneumoniae MH-F agars (Thermo Fisher Scientific) were used as recommended by EUCAST. MH(-F) agars were incubated for 16 h in WASPLab® automated incubators (aerobic for MH-agars and CO2-enriched for MH-F agars). Subsequently, images were acquired, and inhibition zones were read using WASPLab® Webapp [15]. The inhibition zones were manually adjusted when necessary.
Antimicrobials
Two antibiotic panels were used, the first panel targets Gram-negative (GN) bacteria and the second panel targets Gram-positive (GP) bacteria.
Gram-Negative Bacteria
The GN antibiotic panel contained 18 different antibiotics: ampicillin, amoxicillin-clavulanic acid, piperacillin-tazobactam, temocillin, cefadroxil, cefuroxime, ceftriaxone, ceftazidime, cefepime, meropenem, moxifloxacin, ciprofloxacin, amikacin, gentamicin, aztreonam, fosfomycin, nitrofurantoin, sulfamethoxazole/trimethoprim.
Gram-Positive Bacteria
The GP antibiotic panel contained 16 different antibiotics: penicillin, oxacillin, ampicillin, cefoxitin, erythromycin, clindamycin, tetracycline, gentamicin (10 µg and 30 µg), vancomycin, nitrofurantoin, linezolid, rifampicin, moxifloxacin, ciprofloxacin and sulfamethoxazole/trimethoprim.
Thermolability
According to the manufacturer’s instructions, i2a discs should be stored in a dry place between 2 and 25 °C for a maximum of 35 days, except for specific antimicrobial disks, such as frequently tested beta-lactam antibiotics, which have a stability of only 7 days. When placed in a dispenser, a desiccant must be added, a step not feasible with the Radian® carrousel. For Thermo Fisher scientific discs, once opened, cartridges should be stored in a container provided with a desiccant and at 2–8 °C. The stability after opening under the described conditions is specified on the carton for each antimicrobial disc and is maximum 7 days.
Thermolability testing was performed for 10 reference strains at 7 consecutive days leaving the disks in the Radian In-Line Carousel at room temperature [6, 16]. AST was performed as described above with Wasplab/Radian® In-Line Carousel using MH agars except for S. pneumoniae and C. jejuni where MH-F agars (Thermo Fisher Scientific) were used. All strains (Microdiagnostics, United States of America; LGC, United Kingdom; DSMZ, Germany; NCTC, United Kingdom; LMG, Belgium) were stored at −80 °C for 5–20 years and were cultured manually on a non-selective blood agar (Thermo Fisher Scientific, Waltham, USA). These agar plates were incubated for 16 ± 2 h in a CO2 incubator (Thermo Fisher Scientific).
Antibiotic disks (Thermo Fisher Scientific) were added to each MH(-F) agar based on the identification of the ATCC® strain according to EUCAST guidelines. Agars were incubated for 16 h in the WASP® automated incubator, capable of incubating the MH-F agars in CO2 enriched environment. For C. jejuni plates were incubated outside the WASP® in a micro-aerophilic environment (Thermo Fisher Scientific). Inhibition zones were read using WASPLab® Webapp, with manual adjustments where necessary [14, 15]. Inhibition zones were evaluated using EUCAST acceptance limits, and potential trends were assessed [16]. This experiment was repeated five times for each strain.
Quality Control
Quality control strains were performed during each run, utilizing the strains recommended for routine quality control by EUCAST, as outlined in their guidance document version 11, January 2021 [17]. All quality control strains were stored at −80 °C for 5–20 years and were cultured manually on a non-selective blood agar (Thermo Fisher Scientific, Waltham, USA). These agar plates were incubated for 16 h in a CO2 incubator (Thermo Fisher Scientific).
Statistical Analysis
Statistical analyses were performed using Microsoft Excel (version 2016, USA). Categorical agreement (CA) was assessed between standardized disk diffusion and Radian® In-Line Carousel using EUCAST 2021 breakpoints.
Results
Antimicrobial Susceptibility Testing
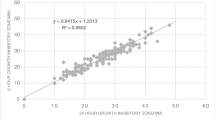
Results of WASPLab® coupled to Radian® In-Line Carousel compared to EUCAST standardized disk diffusion testing are displayed in Table 1.
Enterobacterales
Overall CA of 95.2% was obtained among the 63 Enterobacterales strains that were tested. Within the discrepancies, 4.4% were mD, three were major discrepancies (MD) and one was a very major discrepancy (VMD). Disk diffusion screening cut-off for CPE, according to EUCAST methodology (meropenem inhibition zone diameter < 25 mm), enabled the detection of all CPE isolates. In addition, ESBL screening method according to EUCAST methodology (cefotaxime 5 µg inhibition zone < 21 mm OR ceftriaxone 30 µg inhibition zone < 23 mm AND ceftazidime inhibition zone < 22 mm) allowed detection of 11 ESBL producing isolates using SIRScan® and 12 ESBL producing isolates using Radian® coupled to WASPLab®.
Pseudomonas aeruginosa
An overall CA of 93.8% between both methods was obtained for the 16 P. aeruginosa isolates, with all discrepant results being mD. Disk diffusion screening cut-off for CPE, according to EUCAST methodology (meropenem inhibition zone diameter < 25 mm), enabled the detection of all three MBL producing P. aeruginosa strains.
Enterococcus spp.
Among the 13 Enterococcus spp. isolates a CA of 96.4% was obtained between both methods with 1.2% mD. One MD was observed in a VanB + E. faecalis isolate for vancomycin. However, this appeared to be a correct identification of the VRE strain by Radian® Coupled to WASPLab and a false susceptibility for vancomycin on SIRScan® (external quality control strain). All other VRE isolates were categorized correctly using both methods.
Staphylococcus spp.
Overall CA of 97.3% was obtained with 2.4% mD and one MD. All MRSA isolates were categorized correctly using EUCAST methodology (cefoxitin 30 µg inhibition zone diameter < 22 mm).
Streptococcus spp.
Overall CA of 98.0% was obtained with all discrepant results being mD.
Thermolability of Antimicrobial Disks
Table 2 displays the minimum and maximum amount of days for which the antibiotic disk inhibition zones remained within the expected range when stored in the Radian® carousel. Additional graphs can be found in supplementary material (Fig. S1–S10).
Escherichia coli ATCC® 25922™ and 35218™
All antibiotic disks were stable for at least 4 days for both ATCC® strains. A slight downwards trend was observed for meropenem after 5 days in ATCC® 25922™. For amoxicillin-clavulanic acid, a downwards trend was noticed after 5 days in ATCC® 35218™.
Klebsiella pneumoniae ATCC® 700603™
All antibiotic disks were stable for at least 5 days.
Pseudomonas aeruginosa ATCC® 27853™
All antibiotic disks were stable for at least 5 days. A slightly downwards trend was observed for meropenem after 5 days.
Staphylococcus aureus ATCC® 29213™ and NCTC® 12493™
For ATCC® 29213™, disk diffusion zones for ampicillin and penicillin did not match the EUCAST criteria already on day 1 in all five runs, resulting in a stability of 0 days. During the first run erythromycin only had a stability of 2 days and tetracycline of 0 days. The following runs showed a minimum stability of 4 days for erythromycin and 6 days for tetracycline. Therefore, the first run was discarded. All other antibiotics were stable for at least 4 days. NCTC® 12493™ showed stability of 5 days for all investigated antibiotic disks.
Enterococcus faecalis ATCC® 29212™ and 51299™
All investigated antibiotic disks were stable for at least 4 days.
Streptococcus pneumoniae ATCC® 49619™
All investigated antibiotic disks were stable for at least 4 days.
Campylobacter jejuni ATCC® 33560™
All investigated antibiotic disks were stable for 7 days.
Discussion
This study is the first to compare EUCAST standardized disk diffusion testing using SIRScan® (i2a) with the fully automated WASPLab® setup from Copan, including the Radian® in-Line Carousel. Additionally, the study investigates the thermolability of antibiotic disks when stored at room temperature for up to 7 days.
Until recently, disk diffusion AST was mainly a manual process with the option to use semi-automated reader instruments. The availability of automated liquid-based systems, such as VITEK® 2 (Biomérieux) and Phoenix™ (BD), and the amount of manual work have limited the use of disk diffusion in routine clinical microbiology laboratories. Dauwalder et al. described the automation of disk diffusion as being one of the last pieces missing for full microbiology laboratory automation [7]. Automating the system would expand the possibility of using disk diffusion as a reference method in many laboratories by reducing the workload. Despite its limitations, disk diffusion has several advantages over liquid-based systems. One significant advantage is its greater reliability in detecting heteroresistant profiles and certain carbapenemases. In 2019, Cherkaoui et al. performed an evaluation of the Copan WASPLab incorporating the BioRad expert system against SIRscan 2000, using 388 clinical strains. The inoculation method performed by WASPLab during this study mirrored the inoculum preparation utilized in the current study. Their findings demonstrated an accuracy that was equal or even better than the SIRscan 2000 [18]. However, the current study used the Radian® In-Line carousel for placement of the antimicrobial discs. Furthermore, we included a broader spectrum of resistant strains, such as CPE and multidrug resistant P. aeruginosa. In a subsequent study performed by Cherkaoui et al., the VITEK® 2 system was compared with automated disk diffusion using Radian® In-Line carrousel and found that the main cause of very major errors on the VITEK® 2 was due to heteroresistant populations of P. aeruginosa [3]. The advantage of disk diffusion is the visualization of these heteroresistant populations and antibiotic interactions.
An acceptable overall CA was obtained for all groups. Very major error rates were ≤ 5% for all antibiotics. Radian® was able to detect all CPE strains and all ESBL producing Enterobacterales strains using screening breakpoints provided by EUCAST for ESBL and CPE detections. This is in line with the results obtained by Cherkaoui et al. [3]. Every MBL producing P. aeruginosa was detected using CPE screening breakpoints provided by EUCAST. All VRE and MRSA strains were categorized correctly using EUCAST guidelines.
During the thermostability study, questionable results were obtained for S. aureus ATCC® 29213™ for ampicillin and penicillin disks. The disk diffusion inhibition zones were below the allowed range from day 1. This problem was addressed to the corresponding companies, and we chose not to include this in our study since the issue only occurred with this specific reference strain, and both antibiotics are not used to treat S. aureus infections and are not reported to the clinicians in routine. As of yet, the cause of this problem is still unidentified. Overall, thermostability of the antibiotic disks was verified for at least 4 days when stored at room temperature in the WASP Radian® carousel, facilitating the full automation of AST. While some manufacturers, such as MAST, provide longer stability periods for their antimicrobial discs, it is essential to validate stability within Radian® In-Line carousel. This validation is necessary as the relative humidity within the carousel may differ from that within a manufacturer-provided dispenser with desiccant.
Overall, automation of disk diffusion reduces the workload for laboratory technicians, as manual streaking of MH-plates, placement of antimicrobial disks, and measurement and interpretation of the inhibition zones is time consuming and require significant hands-on time. In contrast, minimal hands-on time is needed for loading the WASP and Radian® carousel, the inhibition zones are measured automatically and require sporadic manual adjustments.
A possible limitation of this study is the relatively small amount of isolates included. However, these isolates were selected to test the capability of the system. They include a challenge panel of bacterial strains selected by the Belgian national antimicrobial susceptibility testing committee [13]. This panel consists of 14 Gram-negative and 14 Gram-positive bacteria covering most important resistance mechanisms and showing stable susceptibility results with both micro-dilution methods and disk-diffusion methods. In addition, we selected isolates from national quality control surveys with known resistance mechanisms. An additional potential limitation is the use of antimicrobial discs from two different manufacturers for disk diffusion by SirScan versus Radian® during this evaluation. As highlighted in a study from EUCAST, variations in the quality of disks exist between different manufacturers. However, in their 2017 evaluation, the discs from SirScan (i2a) and Oxoid (Thermo Fisher Scientific), used during this study, demonstrated comparable performance [19].
Conclusion
In conclusion, this study demonstrates that the Radian® In-Line carousel is a reliable and cost-effective automated disk diffusion method. This method is also flexible and enables visualization of antibiotic interactions. The full automation of AST using disk diffusion provided by Copan may facilitate the implementation of this technique in routine clinical microbiology laboratories.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Banerjee R, Humphries R (2021) Rapid antimicrobial susceptibility testing methods for blood cultures and their clinical impact. Front Med (Lausanne). https://doi.org/10.3389/fmed.2021.635831
Herroelen PH, Heestermans R, Emmerechts K, Vandoorslaer K, Wybo I, Piérard D, Muyldermans A (2022) Validation of Rapid Antimicrobial Susceptibility Testing directly from blood cultures using WASPLab®, including Colibrí™ and Radian® In-Line Carousel. Eur J Clin Microbiol Infect Dis 41:733–739
Cherkaoui A, Renzi G, Vuilleumier N, Schrenzel J (2021) Performance of fully automated antimicrobial disk diffusion susceptibility testing using Copan WASP Colibri coupled to the radian in-line carousel and expert system. J Clin Microbiol. https://doi.org/10.1128/JCM.00777-21
Behera B, Anil Vishnu GK, Chatterjee S, Sitaramgupta VVSN, Sreekumar N, Nagabhushan A, Rajendran N, Prathik BH, Pandya HJ (2019) Emerging technologies for antibiotic susceptibility testing. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2019.111552
Khan ZA, Siddiqui MF, Park S (2019) Current and emerging methods of antibiotic susceptibility testing. Diagnostics. https://doi.org/10.3390/diagnostics9020049
The European Committe on Antimicrobial Susceptibility testing (2021) Antimicrobial susceptibility testing EUCAST disk diffusion method, Version 9.0
Dauwalder O, Vandenesch F (2020) Disc diffusion AST automation: one of the last pieces missing for full microbiology laboratory automation. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2020.01.021
Matuschek E, Brown DFJ, Kahlmeter G (2014) Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. https://doi.org/10.1111/1469-0691.12373
Medeiros AA, Crellin J (2000) Evaluation of the Sirscan automated zone reader in a clinical microbiology laboratory. J Clin Microbiol. https://doi.org/10.1128/JCM.38.4.1688-1693.2000
Zimmermann S (2021) Laboratory automation in the microbiology laboratory: an ongoing journey, not a tale? J Clin Microbiol. https://doi.org/10.1128/JCM.02592-20
Cherkaoui A, Renzi G, Vuilleumier N, Schrenzel J (2019) Copan WASPLab automation significantly reduces incubation times and allows earlier culture readings. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2019.04.001
Cherkaoui A, Renzi G, Viollet A, Fleischmann M, Metral-Boffod L, Dominguez-Amado D, Vuilleumier N, Schrenzel J (2020) Implementation of the WASPLab™ and first year achievements within a university hospital. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-020-03872-1
Desmet S, Verhaegen J, Glupzcynski Y, van Eldere J, Melin P, Goossens H, Piérard D, Declercq P, Lagrou K, Boel A, Cartuyvels R, Denis O, Vandewal W, Saegeman V (2016) Development of a national EUCAST challenge panel for antimicrobial susceptibility testing. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2016.05.011
The European Committee on Antimicrobial Susceptibility testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0, 2021
Copan WASP AST Module - operator manual HPAWAEN REV04 (2021)
The European committee on Antimicrobial Susceptibility Testing (2021) Routine and extended internal quality control for MIC determination and disk diffusion as recommended by EUCAST
The European Committee on Antimicrobial Susceptibility testing. QC tables. Version 11.0, 2021
Cherkaoui A, Renzi G, Fischer A, Azam N, Schorderet D, Vuilleumier N, Schrenzel J (2020) Comparison of the Copan WASPLab incorporating the BioRad expert system against the SIRscan 2000 automatic for routine antimicrobial disc diffusion susceptibility testing. Clin Microbiol Infect 26(5):619–625. https://doi.org/10.1016/j.cmi.2019.11.008
Åhman J, Matuschek E, Kahlmeter G (2019) The quality of antimicrobial discs from nine manufacturers-EUCAST evaluations in 2014 and 2017. Clin Microbiol Infect 25(3):346–352
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The open acces publishing fee was funded by Copan.
Author information
Authors and Affiliations
Contributions
Conceptualization: AM, DP, KE, KC. Methodology: AM, KC, KE, KV. Validation: AM, KE, KV. Formal analysis: KC, KE. Investigation: KC, AM, AS. Writing—original draft: KC. Writing—review and editing: AM, DP, IW, TD, DDG, AS, KC. Supervision: AM, DP, IW.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Callebaut, K., Stoefs, A., Emmerechts, K. et al. Evaluation of Automated Disk Diffusion Antimicrobial Susceptibility Testing Using Radian® In-Line Carousel. Curr Microbiol 81, 196 (2024). https://doi.org/10.1007/s00284-024-03710-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03710-z