Abstract
Legionella pneumophila (Lp) is a Gram-negative bacterium found in natural and artificial aquatic environments and inhalation of contaminated aerosols can cause severe pneumonia known as Legionnaires’ Disease (LD). In Brazil there is hardly any information about this pathogen, so we studied the genetic variation of forty Legionella spp. isolates obtained from hotels, malls, laboratories, retail centers, and companies after culturing in BCYE medium. These isolates were collected from various sources in nine Brazilian states. Molecular identification of the samples was carried out using Sequence-Based Typing (SBT), which consists of sequencing and analysis of seven genes (flaA, pilE, asd, mip, mompS, proA, and neuA) to define a Sequence Type (ST). Eleven STs were identified among 34/40 isolates, of which eight have been previously described (ST1, ST80, ST152, ST242, ST664, ST1185, ST1464, ST1642) and three were new STs (ST2960, ST2962, and ST2963), the former identified in five different cooling towers in the city of São Paulo. The ST1 that is widely distributed in many countries was also the most prevalent in this study. In addition, other STs that we observed have also been associated with legionellosis in other countries, reinforcing the potential of these isolates to cause LD in Brazil. Unfortunately, no human isolates could be characterized until presently, but our observations strongly suggest the need of surveillance implementation system and control measures of Legionella spp. in Brazil, including the use of more sensitive genotyping procedures besides ST.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Legionella sp. is a genus composed of aerobic, Gram-negative, and flagellated bacteria, which includes 59 species, three subspecies and more than 70 serogroups, mostly isolated from natural and artificial aquatic environments [1]. Although all species of the genus have pathogenic potential, the specie L. pneumophila (Lp) is responsible for 90% of the cases of legionellosis around the world [2]. These bacteria can cause Legionnaires’ Disease (LD), an atypical and sometimes fatal pneumonia, or Pontiac Fever, a mild self-limiting flulike illness. Both clinical forms occur through inhalation of contaminated aerosols [3].
Although Legionella are present in natural environments, outbreaks of legionellosis are mainly associated with artificial environments, due to their favorable conditions for colonization such as temperatures between 25 °C and 42 °C, stagnation zones, organic contamination and the presence of protozoa, natural hosts of Legionella [4]. Furthermore, production of aerosols by artificial water systems such as cooling towers, spas, and hot spring baths create conditions for access to the human respiratory system, having turned LD into an emerging disease since the seventies [1].
Consequently, surveillance of such artificial aquatic systems for the presence of Legionella is the most important intervention to prevent the disease and the study of these microorganisms allows the understanding of epidemiological factors involved and their pathological potential.
Currently, there are several techniques used for the molecular characterization of Lp, such as Variable Number of Tandem Repeats (VNTR); Pulsed-Field Gel Electrophoresis (PFGE); and Amplified Fragment Length Polymorphism (AFLP). However, the European Working Group for Legionella Infections (EWGLI), recently renamed European Study Group for Legionella Infections (ESGLI), developed a standardized procedure for the molecular typing of Lp, based on the amplification and sequencing of seven genes (flaA, pilE, asd, mip, mompS, proA, and neuA), establishing as such their Sequence-based Type (SBT) [5].
In Brazil, there is hardly information about presence of Legionella species and if any, based only on serotyping, a procedure lacking discriminative power and lacking information on genetic variability. Therefore, this study aims to carry out the molecular characterization of Lp isolates from environmental sources from different Brazilian states through the SBT technique.
Materials and Methods
Sampling
Forty isolates of Legionella spp. were collected during 2015, 2018, and 2019 and kindly provided by Conforlab (São Paulo), a company that provides environmental quality control services. These isolates were obtained by collecting 1 L of water from different sources, locations, cities, and states in Brazil (Table S1). Water samples were collected in hotels (21), mall (1), retail centers (4), laboratories (2), food industry (1), and a cleaning company (1).
Sample Processing and Isolation of Legionella spp.
Identification of Legionella spp. in the water samples was performed in accordance with the parameters established by ISO 11731, which consists of direct inoculation or inoculation after filtration. During the first procedure, a 200 μL aliquot of 1 L sample was inoculated directly into the selective culture medium buffered charcoal yeast extract (BCYE) agar (Sigma-Aldrich, India), supplemented with glycine (3 g/L), vancomycin hydrochloride (1 mg/L), polymyxin B sulfate (80,000 UI/L), and cycloheximide (80 mg/L) (GVPC; Sigma-Aldrich, Brazil), and incubated at 35º C in an atmosphere of 2.5% CO2 for a period of up to 14 days.
For the second procedure, the rest of the collected material was concentrated by filtration on a sterile cellulose membrane with porosity between 0.2 and 0.45 µM and 47 mm of diameter. This membrane was then transferred to a 50 mL tube containing 10 mL of sterile distilled water, 1 mL of 0.2 M HCl added and incubation at RT for 15 min. To neutralize the solution, 1 mL of 0.1 M KOH was added, and 0.1 mL aliquots were inoculated into Petri dishes containing selective BCYE + GVPC medium. The plates were incubated at 35º C in an atmosphere of 2.5% CO2 for up to 14 days.
Confirmation of the presence of members of the genus Legionella spp. was achieved by incubating the colonies in BCYE medium without the addition of L-cysteine or in blood agar medium, as species of this genus only grow in the presence of this amino acid.
DNA Extraction
One or two loops of bacterial mass were transferred to a microtube with 1 mL of sterile water and centrifuged at 15,000×g for 10 min. The supernatant was discarded, and the pellet was resuspended in 100 µL of RNase buffer (Resuspension Buffer with RNase A – R4) and 5 µL of lysozyme (50 mg/mL). The samples were incubated for 10 min followed by a new step of incubation with 500 µL of lysis buffer (L14) and 10 µL of proteinase K at 80 °C for one hour. All solutions except lysozyme are components of the ChargeSwitch® gDNA Mini Bacteria Kit (Invitrogen, California, USA).
After cell lysis, bacterial DNA was purified by binding to the magnetic beads (ChargeSwitch® Kit) (Invitrogen, California, USA) according to the manufacturer’s protocol and submitted to the magnetic rack (MagnaRack™ Magnetic Separation Rack) (Invitrogen™ / Thermo Fisher, California, USA). DNA quantification by nanodrop is listed in supplementary Table S2.
Sequence-Based Typing
Legionella spp. DNA was subjected to SBT technique according to the protocol proposed by ESGLI, which consists of seven genes fragments amplification (Table S2), followed by sequencing of PCR products and analysis by Sequence Quality Tool of SBT-database [5, 6]. The reference sequences and primers used for SBT scheme are summarized in Table S3. For each sample seven PCR reactions was done. The reaction mixture and conditions used were 1 U de Taq polimerase, 1X PCR buffer, 2,5 mM MgCl2 (Taq DNA Polymerase Recombinant™ kit (Invitrogen™, California, USA); 0.2 mM dNTPs, 0.2 mM of primers forward and reverse, and 4 ng/μL of DNA strain, totaling a volume of 50 µL. Amplification by PCR was performed using the Veriti™ 96-Well Fast Thermal Cycler (Applied Biosystems, California., USA) at the following conditions: denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 56,5 °C for 30 s, elongation at 72 °C for 35 s, and final elongation at 72 °C for 5 min. PCR products were analyzed by agarose gel electrophoresis. As a positive control DNA from strain L. pneumophila ATCC 33152 was used, and PCR products were purified using the ChargeSwitch™ PCR Clean-Up™ Kit (Invitrogen). Both strands of the amplicons were sequenced with a model ABI 3730xL sequencer (Applied Biosystems).
Sequence files were submitted and evaluated by the Sequence Quality Tool (SQT) with the help of Dr. Baharak Afshar (European Program for Public Health Microbiology—ECDC). This tool submits the sequences to a second step of control quality and defines the alleles of the seven before mentioned targets for identification of ST. Strains with known allelic profiles were assigned to a corresponding ST in SBT-database, while strains with new alleles or new allelic profiles were assigned to new STs by database curation. Additionally, sequences obtained were also shared on NCBI Sequence Read Archive (SRA) portal and can be accessed using the BioProject code: PRJNA1068997.
Population Genetics Analysis
After being introduced into an Excel table, the identified STs and the partial allelic profiles were grouped into a dendrogram to verify similarity level between the genotypes. The genetic population structure of the strains was defined by creating a minimum spanning tree (MST) based on allele profiles by using Bionumerics version 7.1 (bioMérieux, Belgium) by creating a similarity matrix using the categorical similarity coefficient. Although this procedure is not the classical sequence analysis MLST based approach with input of complete sequences, we observe that there is an overall ST proximity between isolates based on their alleles.
Results
Samples
Forty isolates of Legionella sp. (Table S1) were obtained from water samples collected from several sources: faucets (35%), cooling towers (22.5%), showers (20%), boilers (12.5%), a drinking fountain (2.5%), and a water reservoir (2.5%). The source of two other samples (5%) is unclear. Among the 40 samples, seven were isolated in the year 2015, 19 in 2018, and 14 in 2019 (Table S1).
Although isolates were obtained in nine Brazilian states, including São Paulo (SP), Rio de Janeiro (RJ), Minas Gerais (MG), Paraná (PR), Rio Grande do Sul (RS), Sergipe (SE), Pernambuco (PE), Bahia (BA), and Goiás (GO); 50% of samples were from SP (Table 1). About 70% from the Southeast region of country, where SP state is located, followed by the Northeast region (15%), South region (12.5%), and only one isolated from the Center-West region of Brazil (2.5%). We did not receive isolates from Northern Brazil.
Sequence-Based Typing
ST is defined from the amplification of seven alleles (Fig. 1) followed by sequencing and analysis by SQT (Figs. S1, S2). It was possible to determine the complete allelic profile for 34 of the 40 isolates and among these, 11 STs were observed (Table 2). Eight had been previously described (ST1, ST80, ST152, ST242, ST664, ST1185, ST1464, and ST1642), while three were new STs (ST2960, ST2962, and ST2963). These three latter did not contain new alleles but were a rearrangement of existing alleles, suggesting the existence of recombination events in the population of L. pneumophila isolates.
PCR standardization using the Lp ATCC 33152 strain for amplification of the seven targets related to the molecular identification of Lp – 1.5% agarose gel in 1X TAE, stained with 0.5 µg/ml ethidium bromide. 1 molecular marker 100 bp ladder; 2 576 bp fragment of the asd gene; 3 414 bp fragment of the flaA gene; 4 559 bp fragment of the mip gene; 5 711 bp fragment of the mompS gene; 6 459 bp fragment of the neuA gene; 7 460 bp fragment of the pilE gene; 8 fragment of 481 bp of the proA gene; and 9 negative control
Although absence of some markers did not allow us to define the ST of six isolates (due to low sequence quality of some alleles), data of the partial allelic profiles obtained are presented in Table 3. Sequences that define each ST are available in NCBI (BioProject code: PRJNA1068997).
Clustered Isolates and Similarity of STs of Legionella pneumophila
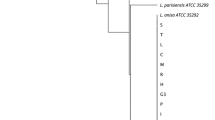
Figure 2 represents the UPGMA based comparison of 34 STs (allelic profile based on seven loci) and the six partially defined profiles. This figure also summarizes the characteristics of isolates.
Result of data clustering obtained by the SBT analysis. In the dendrogram, the columns are represented by the seven alleles, isolate ID, ST, state, origin, and local ID. ST sequence type, N/A ST not defined, CT cooling tower, WT water tank. SE Sergipe, SP São Paulo, MG Minas Gerais, RJ Rio de Janeiro, GO Goiás, RS Rio Grande do Sul, PR Paraná, PE Pernambuco, BA Bahia. Location ID is defined by a letter + number, so: DF = drinking fountains, H = hotel, M = mall, L = laboratory, RC = retail center, and C = company. Allele code “0” means no allele number definition
Among the 40 isolates, 28 (70%) shared their genotype with at least one other isolate and were therefore clustered. The largest cluster was composed by 14 isolates with ST1, followed by ST2960 composed by eight strains. Three small clusters formed by two isolates each had been isolated and included two isolates from different hotels localized in São Paulo city (ST242); two isolates obtained from different sources of a hotel in Paranaguá city (ST664) and two isolates from different sources of the same hotel in Foz do Iguaçu city (ST1464).
Upon analysis of the differences between genotypes, the two largest clusters (ST1 and ST2960) presented difference in a single locus only (neuA), the same being the case for the singleton ST152. Considerable similarity was also observed between ST242, ST1642 and ST664, ST242 and ST1464 presenting only two different loci (pilE and mompS). Higher variability was observed between ST1642 and ST664, presenting a difference in three loci (pilE, mip, and neuA), while ST664 and ST242 share three identical loci only, the same occurring with ST242 and ST1464.
Low genotype similarity was observed comparing ST80, ST1185, and ST2962. ST80 shares two alleles with ST152 (asd and neuA) and shares only the asd allele with ST1 and ST2960. ST1185 shares just flaA allele with ST242, ST664 e ST1642. Finally, the new ST2962 shares neuA allele only with ST1.
When including analysis of incomplete genotypes, the newly described ST2963 presents high similarity with those of incomplete allelic profile of samples 66931 and 66905. Alleles identified in the isolate 66931 are the same as those in ST2963 while among the five defined alleles of sample 66905, four of identical to those defining ST2963.
STs and Partial Allelic Profile Distribution According to State and Source
Table 4 resumes the distribution of STs according to state of origin and showing that isolates from SP state presenting the largest genetic variability, followed by RJ and PR. Among three samples from RJ, two identified STs showing high similarity (ST1 and ST2960); however, the partial profile of 18HP14 isolate indicates a different genotype with considerable similarity to ST664. In Paraná state, the four isolates belong to two totally different STs (ST664 and ST1464). All samples from MG and the only GO strain belong to ST1, while ST80 was identified only in RS sample. Samples 37064 and 37065 were obtained, respectively, from a shower and a sink of the same hotel in Recife (Table S1), but the alleles identified are not the same in these two strains (37064 = 3, 4, 0, 1, 14, 0, 1) and (37065 = 0, 12, 31, 6, 48, 31, 3).
Figure 3 demonstrates the general genotypic distribution and the source dependent genotypic distribution of our sampling. Among 14 isolates obtained from tap water, five STs were identified (ST1, ST80, ST242, ST664, and ST1464) in addition to two partial allelic profiles. For five strains from boilers, four STs were found (ST1, ST152, ST242, and ST1642). Six STs were identified for eight isolates from shower samples (ST664, ST1185, ST1464, ST2960, 2962 and ST2963), in addition to two other partial profiles, showing considerable genetic variability in these sample sources (Fig. 2B). For nine isolates from the cooling towers, seven isolates belong to ST2960 and two have a partial allelic profile like the newly described ST2963 (Fig. 2B). Except for cooling towers, no correlation between source and genotype has been established.
Minimum Spanning Tree based on the STs found (a) and based on the isolates sources (b). STs are represented by circles whose size indicates the number of isolates with this ST. Solid and thick lines connect two STs that differ within a single locus, solid and thin lines connect dual-locus variants, thick dotted lines connect triple-locus variants, and thin dotted lines connect STs that differ at four loci. A N/A = ST not defined, only partial allelic profile; numbers 1 to 6 in the circles refer, respectively, to isolates 66905, 66931, 18HP14, 37064, ISHP03, and 37065. B WT water tank; DF Drinking fountain; CT cooling tower
Discussion
Most of the isolates in the present study were derived from hotels, establishments that are known to solicitate quality of their water facilities more frequently. According to CDC-USA [7], frequent visits in hotels can be a risk factor for legionellosis due to exposure of individuals to possible sources of contamination such as showers, taps, bathtubs, and heaters. Furthermore, it is known that the degree of contamination by Legionella is higher in water systems of large buildings, probably due to the extension of their hydraulic systems [8].
Among the genotypes obtained presently, ST1 is the largest cluster, and composed by samples of five different states collected in 2015, 2018, and 2019. This result seems consistent with data available in literature, where ST1 is a dominant genotype and widely distributed worldwide well adapted to survival in artificial water sources. In addition, organisms with this genotype are considered the main causative agent of LD globally, supporting its high pathogenicity [9,10,11].
The second largest cluster is formed by eight isolates with the new ST2960. One sample was isolated from a shower while seven more isolates were from cooling towers in four different locations: a retail center in RJ and one in SP, and a laboratory and a shopping mall, both in SP. Cooling towers use water as to lower temperature during industrial processes or in buildings with a central air conditioning system. This created humid and hot (30–45 °C) environments with stagnation zones, favoring biofilm development. About 21% of water samples collected from cooling towers in Brazilian companies are contaminated with Legionella spp. (Conforlab, personal communication); such incidence seems to exist in other countries, including in the USA [12].
Because our study was done on a convenience sampling, the fact that 70% of samples share their genotype with one or more isolates calls attention and could be partly explained by the predominance of samples from São Paulo. Additionally, the collection of more than one water sample from the same place, such as hotels and malls, but from different sources such as showers and faucets, could have contributed to the formation of smaller cluster (ST242, ST664, ST1464).
Since this is the first study of Legionella genotyping in Brazil, we included our data of the partial allelic profiles obtained (Table 3) and their participation in the generation of potential new genotypes, adding to genotypic variability. Failure of amplification of some alleles could be due to presence of mutations in primer(s) annealing site or presence of species different from L. pneumophila.
As observed with strains 37063 and 37064, isolates obtained from the same place may present different STs. As shown by Sharaby et al. [13], samples obtained by collecting water from the same distribution system may have different genotypes and, consequently, different characteristics. The diversity of alleles present in a given population is what results in genetic variability of species and can promote differences in genes functionality involved, [14]. Genes encoding flagellum-related proteins (flaA), pilin (pilE), outer membrane protein (mompS), macrophage infectivity enhancer (mip), and zinc metalloproteinase (proA) can interact with external environments; therefore, the adaptation of bacteria to a given environmental source may result in specific and suitable STs for each source, as may have occurred with cooling towers in this study [9]. Furthermore, the findings of this study demonstrate that identical environmental strains can be found at different sampling sites suggest the existence of a complex environmental network that needs further investigation.
Upon comparing our data with those described in literature, eight STs identified presently have been previously described in other countries and some have clearly been associated with Legionnaires’ Disease (Table 5). Isolates with ST1464 have been observed in environmental sources in Indonesia, China, and India, but are apparently not associated with clinical cases in these countries [9]. However, ST664 was associated with two cases of LD in Belgium; one case of unknown origin and another case of travel-associated pneumonia [15].
ST242 genotype was also described in environmental samples from natural and artificial sources in China and in engineered water systems in Japan [16,17,18]. Interestingly, in Arizona (USA), Japan, and United Kingdom, this allelic profile was observed in clinical samples [16, 19]. ST152 has been observed in water samples from two hospitals in Poland in 2001 and 2005. According to Pancer [20], this ST is frequently seen in strains from hospital environmental sources and in clinical samples from patients with CAP or travel-associated pneumonia.
Isolates with ST1642 have been obtained in hotel water system samples in Israel and are widely distributed in the country just like ST1 [21]. Finally, ST80 was also found in Sweden and is among the most common STs among environmental samples in the country; however, no information is available whether they are associated with clinical cases [22].
It is important to note that although this study exclusively analyzed environmental isolates, the genotypes identified here are also associated with cases of legionellosis in other countries, reinforcing the clinical importance of these samples.
Interestingly, the fact that the new STs (not counting the partial allelic profiles that indicate the probability of other new STs) represent 27% of isolates suggests that a considerable number of so far undescribed Lp genotypes might be present in Brazil´s huge territory. Therefore, recombination events might occur frequently in Lp and this suggests the need to continue monitoring L. pneumophila genotypes as recombination events can also affect the adaptability, transmission, and pathogenicity of the isolates.
The major limitation of our study is the relatively small number of isolates that could be genotyped, all being environmental and the majority being from the SP state resulting in a disproportionate distribution among Brazilian states. Nonetheless, this is the first molecular identification study performed with Brazilian isolates and, therefore, may be useful for a timely characteristic’s prediction of isolates in environment before being evidenced in clinical samples.
Conclusion
Monitoring water quality in distribution systems is essential to verify presence of Lp in environment and is the main way to prevent LD. Several countries have strict surveillance for legionellosis, but is absent in Brazil, where control exist only by quality control of water-cooling system upon solicitation and without further notification to the health system. We here present the genetic characterization of Legionella in several water samples in nine Brazilian states and the frequency of STs found, including the presence of genotypes of clinical importance in other countries. Furthermore, a considerable number of isolates presented new STs showing that the genetic variability of Lp is larger that so far described. We also reinforce the need to develop more specific water quality standards for the control and prevention of legionellosis, in addition to the implementation of diagnostic and treatment methodologies in country.
Data Availability
Data will made available on request. The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
References
Khodr A, Kay E, Gomez-Valero L et al (2016) Molecular epidemiology, phylogeny and evolution of Legionella. Infect Genet Evol 43:108–122. https://doi.org/10.1016/j.meegid.2016.04.033
Burillo A, Pedro-Botet ML, Bouza E (2017) Microbiology and epidemiology of legionnaire’s disease. Infect Dis Clin North Am 31:7–27. https://doi.org/10.1016/j.idc.2016.10.002
Mercante JW, Winchell JM (2015) Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev 28:95–133
Buchrieser C, Hilbi H (2013) Legionella: methods and protocols. Humana Press, Totowa
Gaia V, Fry NK, Afshar B et al (2005) Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J Clin Microbiol 43:2047–2052. https://doi.org/10.1128/JCM.43.5.2047-2052.2005
Ratzow S, Gaia V, Helbig JH et al (2007) Addition of neuA, the gene encoding N-acylneuraminate cytidylyl transferase, increases the discriminatory ability of the consensus sequence-based scheme for typing Legionella pneumophila serogroup 1 strains. J Clin Microbiol 45:1965–1968. https://doi.org/10.1128/JCM.00261-07
Center for Disease Control and Prevention (2021) Legionnaires disease cause and spread | CDC. In: Legionella (Legionnaires’ Disease and Pontiac Fever). https://www.cdc.gov/legionella/about/causes-transmission.html. Accessed 24 Jan 2022
Garner E, Brown CL, Schwake DO et al (2019) Comparison of whole-genome sequences of Legionella pneumophila in tap water and in clinical strains, Flint, Michigan, USA, 2016. Emerg Infect Dis 25:2013–2020. https://doi.org/10.3201/eid2511.181032
Sreenath K, Chaudhry R, Vinayaraj EV et al (2020) Distribution of virulence genes and sequence-based types among Legionella pneumophila isolated from the water systems of a tertiary care hospital in India. Front Public Health. https://doi.org/10.3389/fpubh.2020.596463
Beatson SA, Bartley PB (2017) Diving deep into hospital-acquired Legionella pneumophila with whole-genome sequencing. Clin Infect Dis 64:1260–1262. https://doi.org/10.1093/cid/cix156
Wüthrich D, Gautsch S, Spieler-Denz R et al (2019) Air-conditioner cooling towers as complex reservoirs and continuous source of Legionella pneumophila infection evidenced by a genomic analysis study in 2017. Euro Surveill. https://doi.org/10.2807/1560-7917.ES.2019.24.4.1800192
Walser SM, Gerstner DG, Brenner B et al (2014) Assessing the environmental health relevance of cooling towers—a systematic review of legionellosis outbreaks. Int J Hyg Environ Health 217:145–154. https://doi.org/10.1016/j.ijheh.2013.08.002
Sharaby Y, Nitzan O, Brettar I et al (2019) Antimicrobial agent susceptibilities of Legionella pneumophila MLVA-8 genotypes. Sci Rep 9:6138. https://doi.org/10.1038/s41598-019-42425-1
Willard HF, Mcinnes RR, Nussbaum RL (2016) VARIAÇÃO GENÉTICA NAS POPULAÇÕES. In: Thompson & Thompson Genética Médica, 8th ed. Elsevier, New York, p 155
Vekens E, Soetens O, Mendonça R, et al (2012) Sequence-based typing of Legionella pneumophila serogroup 1 clinical isolates from Belgium between 2000 and 2010. Eurosurveillance 6:20302
Guo J, Liang T, Hu C et al (2014) Sequence types diversity of Legionella pneumophila isolates from environmental water sources in Guangzhou and Jiangmen, China. Infect Genet Evol. https://doi.org/10.1016/j.meegid.2014.10.023
Zeng LZ, Liao HY, Luo LZ et al (2019) An investigation on the molecular characteristics and intracellular growth ability among environmental and clinical isolates of Legionella pneumophila in Sichuan Province, China. Biomed Environ Sci 32:520–530. https://doi.org/10.3967/bes2019.069
Zhan X-Y, Zhu Q-Y (2018) Molecular typing of Legionella pneumophila isolates from environmental water samples and clinical samples using a five-gene sequence typing and standard sequence-based typing. PLoS ONE. https://doi.org/10.1371/journal.pone.0190986
Raphael BH, Huynh T, Brown E, et al (2019) Culture of Clinical Specimens Reveals Extensive Diversity of Legionella pneumophila Strains in Arizona. mSphere. https://doi.org/10.1128/mSphere.00649-18
Pancer K (2013) Sequence-based typing of Legionella pneumophila strains isolated from hospital water distribution systems as a complementary element of risk assessment of legionellosis in Poland. Ann Agric Environ Med 20:436–440
Yakunin E, Kostyal E, Agmon V et al (2020) A snapshot of the prevalence and molecular diversity of Legionella pneumophila in the water systems of Israeli hotels. Pathogens 9:414. https://doi.org/10.3390/pathogens9060414
Allestam G, Schönning C (2015) Typning av Legionella på Folkhälsomyndigheten. https://s3-eu-west-1.amazonaws.com/static.wm3.se/sites/16/media/32367_Allestam_Hygiendagarna_i_Ume%C3%A5_150417.pdf?1429645453. Accessed 28 Jul 2021
Acknowledgements
We are grateful to Dr Baharak Afshar for evaluating and assigning the newly identified alleles and STs in this study.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Ethical approval was not needed for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferrari, D.D.d., Lima, S.C., Teixeira, R.L.F. et al. Genetic Diversity of Legionella pneumophila Isolates from Artificial Water Sources in Brazil. Curr Microbiol 81, 165 (2024). https://doi.org/10.1007/s00284-024-03645-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03645-5